A breakthrough approach providing the missing link — ensuring a diverse gut and a strong gut barrier

Addressing gut health challenges
Gut health is essential for overall well-being, yet lifestyle habits, environmental factors such as diet, and medicines contribute to an increasing prevalence of gut dysbiosis and a compromised gut lining. These disruptions can have significant health implications, ranging from acute digestive discomfort to long-lasting challenges and broader systemic effects1. In fact, the U.S. microbiome has lost over 30% of its bacterial diversity — mainly due to antibiotics and poor diet2, 3.
For too long, a critical insight has been overlooked: susceptibility to gut issues is not solely determined by exposure to viruses or harmful bacteria, but by the body's underlying resilience — particularly the strength and stability of the gut microbiome. In other words, external stressors weaken this microbial foundation, and the resulting loss of resilience leaves the gut more vulnerable to disruption.
Traditionally, dietary fibers, fermented foods, probiotics, and prebiotics have been promoted to support gut health. However, new innovations have expanded the range of functional food ingredients available to help maintain a balanced gut. One of the most promising advancements in this field is Binding Proteins. These proteins act as gut-stabilizing ingredients, leveraging natural mechanisms to support gut health and immunity unlocking the option to maintain a healthy gut proactively4.
The science behind Binding Proteins
Binding Proteins are a novel class of functional protein ingredients inspired by nature and the protection from immunoglobulins found in colostrum and raw milk. Binding Proteins, derived from camelid immunoglobulin G (IgG) binding domains, are specifically designed by nature itself to selectively bind and neutralize microbial metabolites that can compromise your gut health. Binding Proteins stop the metabolites from disrupting gut homeostasis, by gently and specifically binding them and allowing a safe passage through your gastrointestinal tract5.

Key properties of Binding Proteins
- Targeted binding Each Binding Protein specifically binds to selected microbial toxins, undesired metabolites, or microbes facilitating their safe and gentle passage through the gastrointestinal tract
- Fast acting Binding Proteins have a fast onset of action with effects observable shortly after ingestion
- Resilient structure Binding Proteins are small (12–30 kDa) and naturally robust against enzymatic degradation, extreme pH, and heat, ensuring stability during food processing, in storage, and in use
- Versatile applications Binding Proteins can be incorporated into various food and supplement formats without altering taste, texture, or nutritional profiles
- Gentle and non-absorptive Binding Proteins do not cross the gut epithelial barrier or activate the immune system, meaning low risk of allergic reactions via dietary exposure
- Safety Binding Proteins are well tolerated, with no evidence of any adverse findings in safety studies and an established NOAEL level of 450 mg/kg body weight/day6

Targeted binding action
Binding Proteins are documented to block enterotoxins completely in a dose dependent manner in gut cell models:
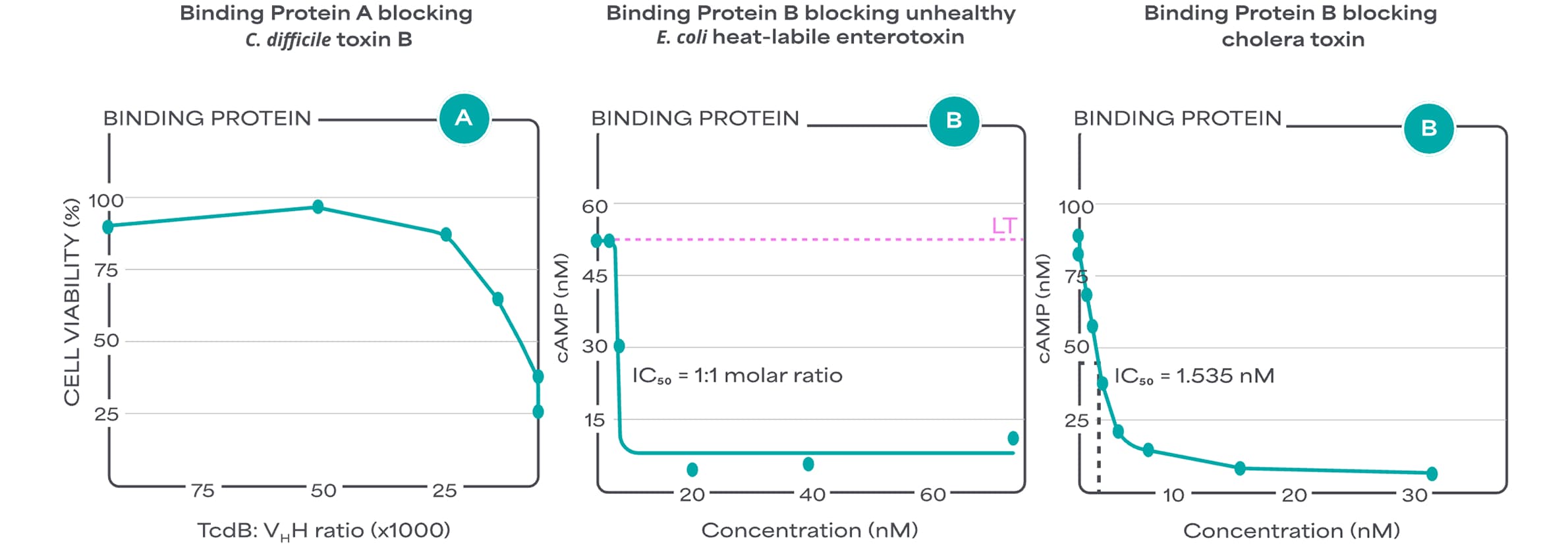
Stability of Binding Proteins in different matrices
Stability measured in a capsule and stick packs:

Robust against extreme pH, enzymatic degradation, and heat
Binding Proteins tested in a pepsin resistance model and a pH and temperature assay:


The gut barrier and its role in health
A well-functioning gut lining acts as the body's first line of defense, preventing harmful substances from entering the bloodstream while allowing essential nutrients to be absorbed. Disruptions to the gut barrier—commonly referred to as "leaky gut" - have been associated with various health challenges, including inflammatory responses and digestive distress8. Emerging research suggests that reinforcing gut barrier integrity through targeted nutritional interventions can provide a proactive approach in supporting long-term gut health9.


Binding Proteins: A novel approach to gut stability
Binding Proteins offer a differentiated approach to gut health by mimicking the natural protective functions of immunoglobulins found in milk and colostrum – they are a specific and convenient daily passive immunity boost. While conventional probiotics or prebiotics focus on promoting microbial gut diversity10, Binding Proteins directly interact with and neutralize microbial risk factors, blocking their harmful effect on your gut, such as inflammation and degradation of the gut barrier (ie. tight-junction proteins), helping to maintain gut homeostasis in a gentle and effective manner5.

Advantages for formulators and brands

Manufacturing – scaling a groundbreaking solution with precision fermentation
Binding Proteins are manufactured using established food industry best practices, including established (GRAS) microbial expression methods, adherence to Good Manufacturing Practices (GMP), and Hazard Analysis and Critical Control Points (HACCP) protocols.
Key specifications of Binding Protein
- Produced in Europe and manufactured via precision fermentation in controlled conditions
- Available as a standardized, off-white powder with high solubility
- Compliant with ISO 9001 quality standards
- Formulated to ensure long-term stability across various food matrices and in applications such as capsules, sachets, and powder
The history and discovery of Binding Proteins
Immunoglobulins (a family of globular proteins with protective bioactivities) are among the most important components of all types of milks and colostra, including those from humans, cows, goats, sheep, and camels11–14. Particular attention has been given to the potential health benefits from the ingestion of camel milk. Camel milk proteins have been credited anti-diabetic, anti-cholesterol, autism alleviating, and antimicrobial properties14–19. Camels have acquired significant adaptations enabling them to thrive in particular harsh climates and therefore possess a key role in societies in arid and semi-arid regions where their milk has been a part of human nutrition since ancient time20,21.
Uniquely, it was discovered in 1993 that immunoglobulins from camelids (camels, alpacas, lamas etc.) have a simple structure, and a binding domain comprising a single polypeptide chain, making it a small stable protein with a targeted binding activity. This discovery has since been leveraged to develop ground-breaking new products, where the power of immunoglobulins can be concentrated, scaled, and embedded in everyday food as functional Binding Proteins.
Key milestones in the discovery of Binding Proteins

A next-generation ingredient for gut health
As consumer demand for scientifically validated gut health solutions continues to grow, Binding Proteins represent a pioneering approach to maintain gut stability. With their targeted mode of action, non-immunogenic nature, stability during processing, and versatility in application, Binding Proteins offer an innovative solution for brands developing next-generation gut health products.
References
- Illiano, P. et al. (2020) FEBS Journal 287.
- Holmberg, S. M. et al. (2024) Nature Communications 15.
- Francino, M. P. (2016) Frontiers in Microbiology 6.
- Petersson, M. et al. (2023) Trends in Biotechnology 41.
- Rodriguez Rodriguez, E. R. et al. (2024) Protein Science 33.
- Phipps, K. R. et al. (2025) Journal of Applied Toxicology.
- Fiil, B. K. et al. (2022) iScience 25.
- Camilleri, M. (2019) Gut 68.
- Zhang, P. (2022) International Journal of Molecular Sciences 23.
- Aziz, T. et al. (2024) Gut Microbes 16.
- Farooq, U. et al. (2024) Animal Biotechnology 35.
- Hurley, W. L. & Theil, P. K. (2011) Nutrients 3.
- Kessler, E. C. et al (2019) Journal of Dairy Science 102.
- Swelum, A. A. et al. (2021) Saudi Journal of Biological Sciences 28.
- Jrad, Z. et al. (2014) Dairy Science and Technology 94.
- Alavi, F. et al. (2017) Nutrients in Dairy and their Implications on Health and Disease, pp. 451–468.
- Hailu, Y. et al. (2016) Journal of Dairy Research 83.
- Kumar, D. et al. (2016) Nutrition and Food Science 46.
- Abd El-Aziz, M. et al (2022) Egyptian Journal of Chemistry 65.
- El-Hatmi, H. et al. (2007) Small Ruminant Research 70, pp. 267–271.
- Cheikh Ismail, L. et al. (2022) Journal of Nutritional Science 11.
- Jenkins, T.P. et al. (2024) npj Biofilms and Microbiomes, 10, 42.
- Farah, Z. (1993) Journal of Dairy Research, 60(4), 603-626.
- Elagamy, E. I. et al. (1992). Journal of Dairy Research, 59(2), 169–175.
- Hamers-Casterman, C. et al (1993) Nature, 363(6428), 446–448.
- Cook, D. et al. (2010) Toxicon, 56(4):596-603
- Grove, S. et al. (2023), Seges Innovation, nr. 1281.
- Peterson, M. et al. (2025) Nature Communications 16, 2722



.png)