Gut Dysbiosis Uncovered: How Gut Diversity & Gut Barrier Function Play a Crucial Role in Maintaining Your Health

Microbial imbalance and immune disruption
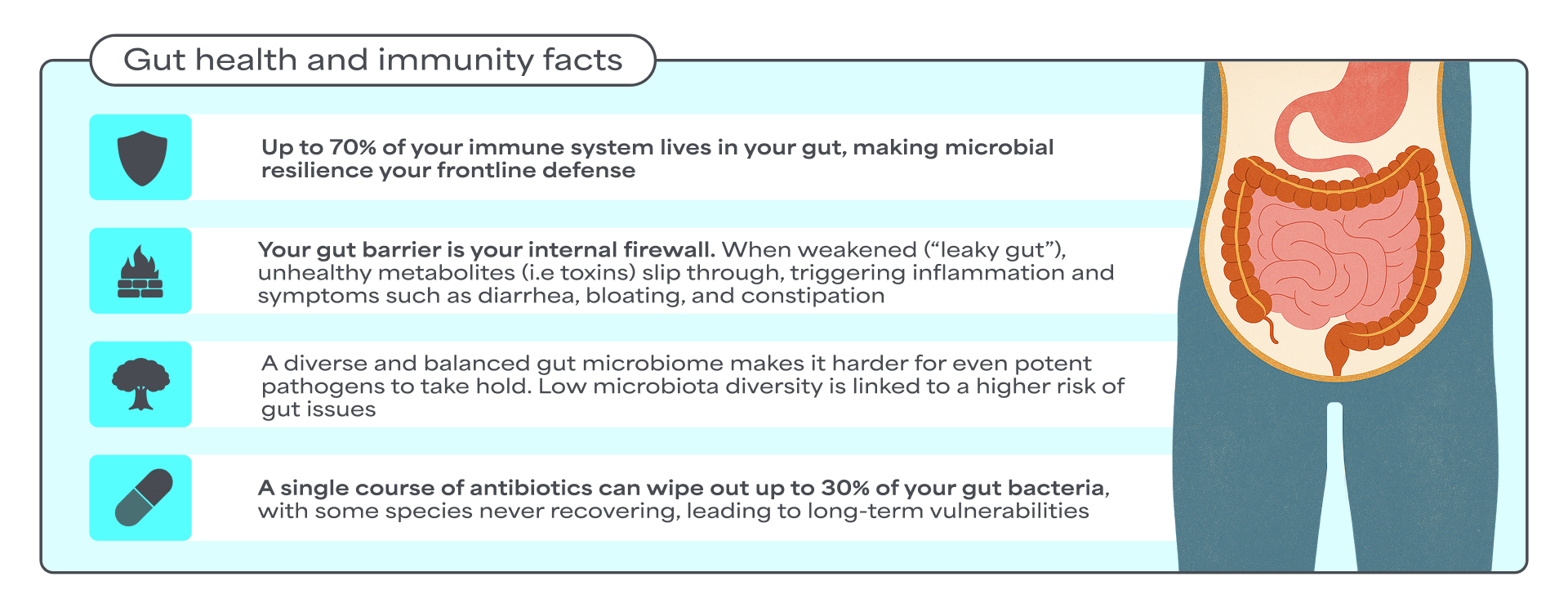
The human gut microbiota plays a pivotal role in maintaining overall health. When the composition and function of this microbial ecosystem become imbalanced, we talk about gut dysbiosis. This imbalance contributes to a dysregulated gut-immune axis, referring to impaired communication and feedback loop between the gut microbiota, intestinal barrier, and the immune system1.
Although the exact prevalence of gut dysbiosis in the general, asymptomatic population is scarce, studies have shown an association between gut microbiota imbalances and various pathological conditions such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), obesity, and allergic disorders2.
The importance of microbiota diversity
A key indicator of gut health
A diverse gut microbiota is a hallmark of gut health. The human gut harbors trillions of microorganisms, predominantly bacteria, but also fungi, protists, archaea, and viruses. This microbial ecosystem plays a crucial role in human physiology and health, collectively aiding digestion, synthesizing essential nutrients, and regulating immune responses3.
External stress factors, such as extreme dietary changes, infections, or antibiotic use, can disrupt this microbial ecosystem. Several human intervention studies have shown that dietary fiber intake increases gut microbiota diversity4. Conversely, other studies have found that antibiotic administration decreases microbiota diversity, often leading to incomplete restoration of microbial composition5.
Decrease of microbiota diversity can cause alteration in the abundance of bacterial-produced metabolites such as short-chain fatty acids (SCFAs). SCFAs are one of the most important metabolite categories involved in the regulation of several biological functions, playing a key role in supporting a resilient and diverse gut microbiota. To promote SCFA production, the diet should be rich in dietary fibers found in plant-based foods such as fruits, vegetables, legumes, and whole grains.
The gut barrier
A critical defense system
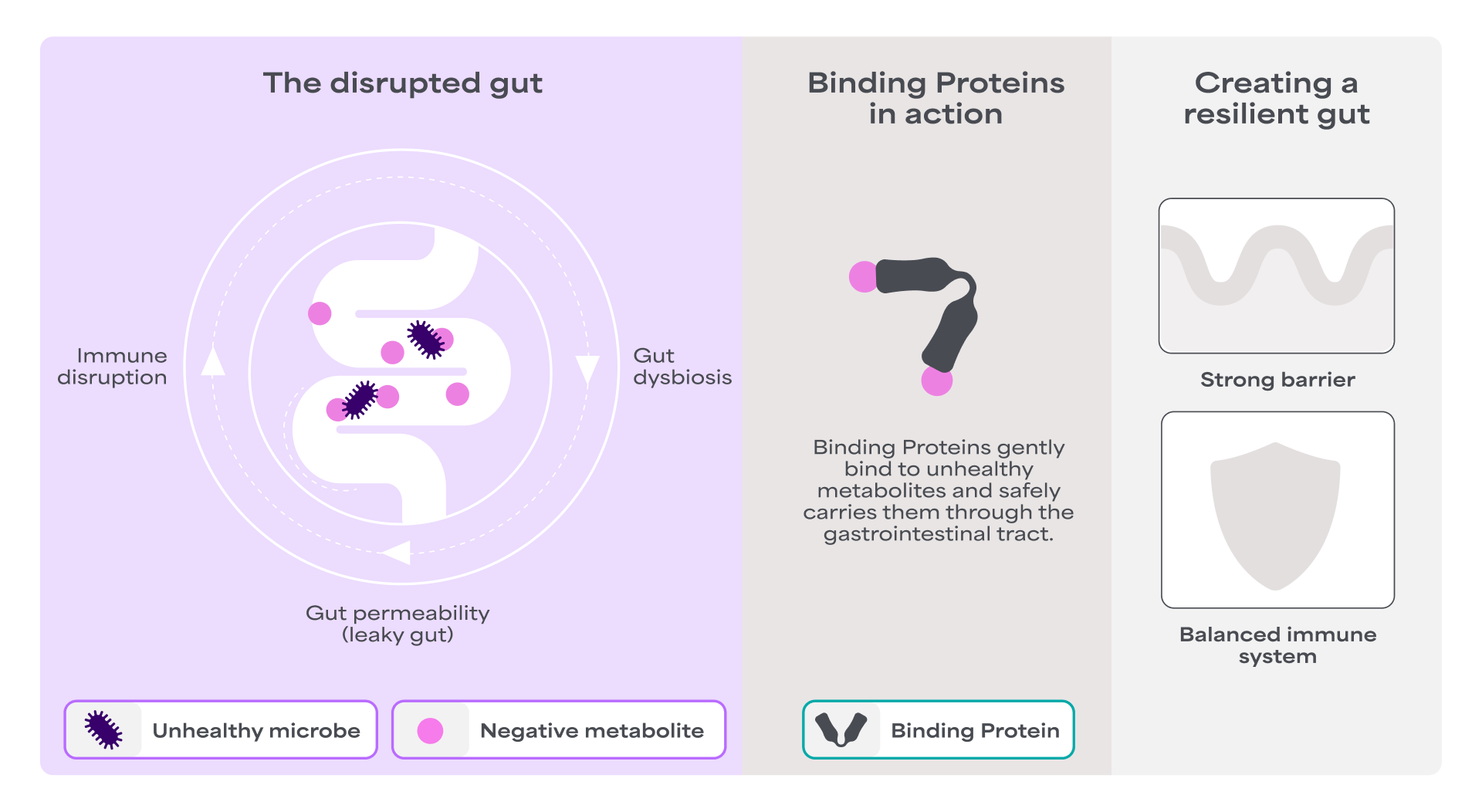
The intestinal mucosal barrier, also referred to as the gut barrier, is widely recognized as a critical player in the gut-immune axis as it ensures the complex crosstalk between the gut microbiota (both commensals and pathogens) and the host immune system. The gut barrier is a physical and biochemical barrier that regulates the selective permeability of the gut.
The gut barrier acts like a smart gatekeeper for your intestines. It helps keep your gut balanced by letting in the good stuff, like nutrients and blocking out harmful substances such as bacteria and toxins. This careful control helps maintain a healthy gut environment, which is called gut homeostasis7.
One important factor that affects the gut barrier is the gut microbiota. An in vivo study has shown that the gut microbiota can directly influence how "leaky" the gut barrier becomes. The study showed that high abundance of unhealthy bacteria, such as Proteobacteria (incl. E. coli), was associated with a disrupted gut barrier. This suggests that shifts in microbiota composition can compromise the gut barrier function8. In contrast, certain good bacteria, like Bifidobacterium, have been shown to reduce inflammation and help strengthen the gut barrier9.
Key consequences linked to reduced microbiota diversity and a disrupted gut barrier
- Reduced microbiota diversity can decrease the production of essential metabolites like SCFAs, leading to gut dysbiosis.
- Dysbiosis can increase the presence of pathogenic bacteria in the gut and reduce gut resilience.
- Reduced microbiota diversity and disrupted gut barrier integrity are associated with various pathological conditions such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and food allergies.
- Disrupted gut barrier function allows harmful substances to pass into the bloodstream. Once in the blood, these substances (e.g., microbial toxins, dietary antigens, or heavy metals) can trigger the body's immune system and cause widespread inflammation, which over time may lead to long-term diseases.
The rise of consumer demand for gut-supporting solutions

Enter Binding Proteins
The next frontier in gut health
Binding Proteins: A novel class of functional ingredients derived from camelid immunoglobulin G (IgG) binding domains, inspired by the protective properties of immunoglobulins naturally found in colostrum and raw milk that has a long history of safe use. They are very target specific, designed to selectively bind and neutralize microbial-derived risk factors10,11.
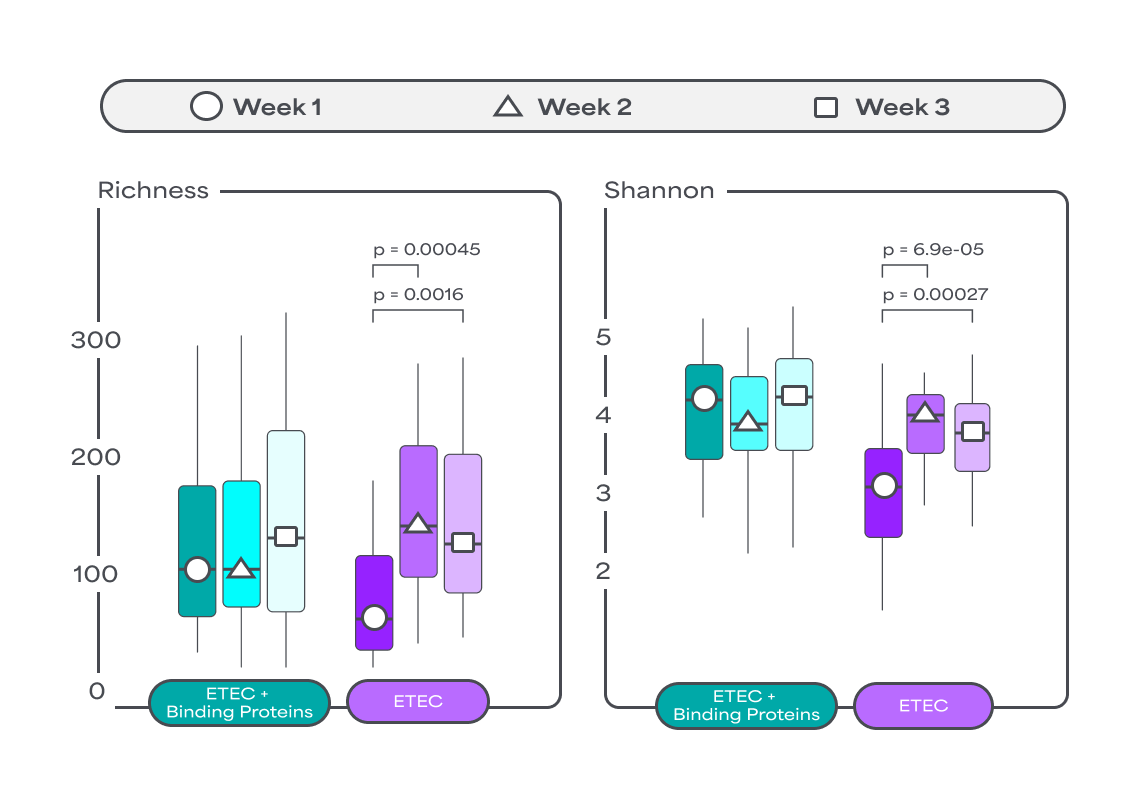
Binding Proteins have been shown to positively impact the gut microbiota by accelerating the recovery of microbiota diversity and induce lower abundance of unhealthy bacteria in vivo12. By fostering a balanced microbial environment, Binding Proteins can help reduce the need for antibiotics, which, although effective, can negatively impact the gut microbiota diversity.
Supports a healthy gut barrier
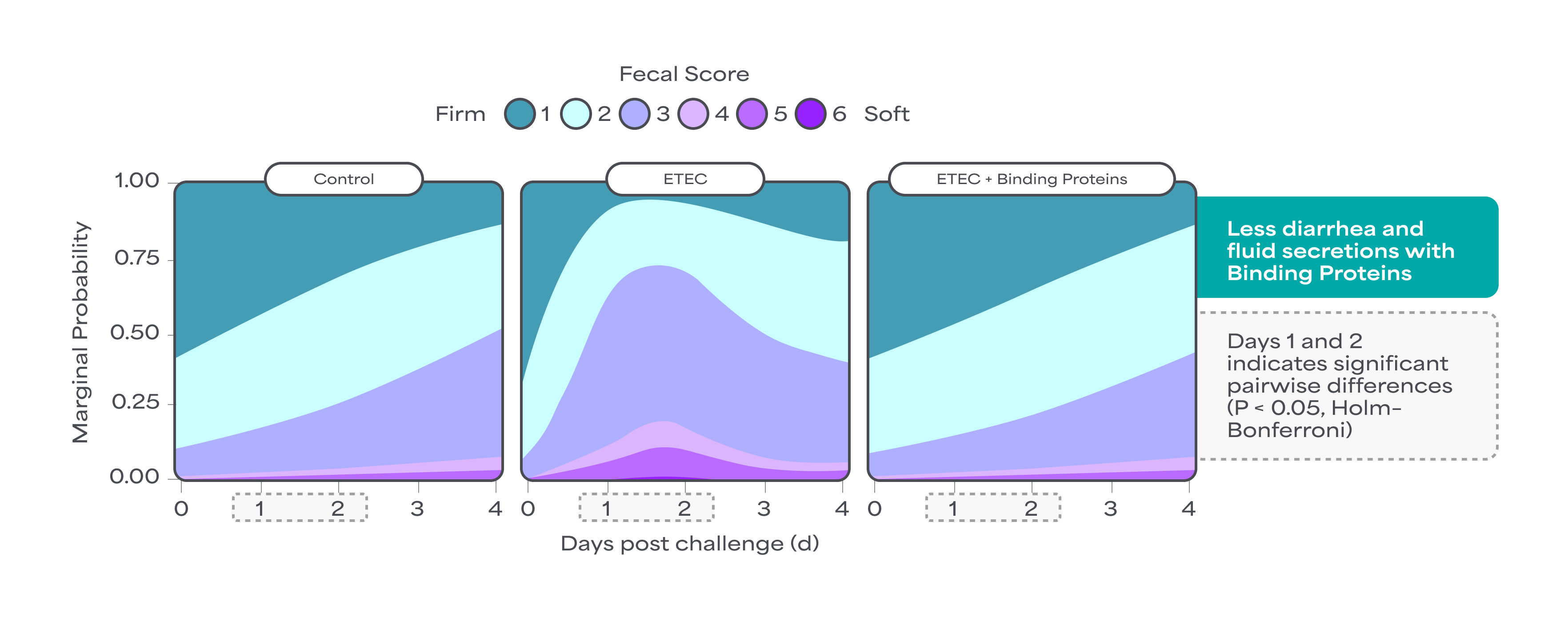
Binding Proteins help maintain the normal function of the gut lining. An in vivo study has demonstrated that they significantly contribute to the gut defense system by enhancing gut integrity under challenges, such as ETEC infection. This without inducing inflammation and preserving small intestinal tight junction levels15.

Nurturing microbiota diversity
Binding Proteins have been shown to positively impact the gut microbiota by accelerating the recovery of microbiota diversity and induce lower abundance of unhealthy bacteria in vivo 12.
Maintains hydration & electrolyte levels
Binding Proteins contribute to the gut defense system. Both in vitro and in vivo data demonstrate that they prevent the entry of harmful metabolites, such as bacterial toxins, into intestinal cells by binding to harmful metabolites. This binding neutralizes the destructive activity. Data further show that Binding Proteins can help maintain intestinal integrity and contribute to the regulation of gut fluid and electrolyte balance. This regulation supports the reduction of diarrhea and excessive fluid secretion, highlighting their capacity to promote fluid and electrolyte homeostasis13,14.
References
- Stolfi, C. et al. (2022) Biomedicines 10.
- Duan, H. et al. (2022) Critical Reviews in Food Science and Nutrition 62
- Qin, J. et al. (2010) Nature 464.
- Cronin, P. et al. (2021) Nutrients 13.
- Pałangia, D. V. et al. (2022) Microbiology Open 11.
- Dogra, S. K. et al. (2020) Frontiers in Microbiology 11
- Wells, J. M. et al. (2017) American Journal of Physiology - Gastrointestinal and Liver Physiology 312
- Jakobsson, H. E. et al. (2015) EMBO Rep 16.
- Ruiz, L. et al. (2017) Frontiers in Microbiology 8.
- Fiil, B. K. et al. (2022) iScience 25.
- Petersson, M. et al. (2024) Trends in Biotechnology 41.
- Jenkins, T. P. et al. (2024) NPJ Biofilms Microbiomes 10.
- Rodriguez Rodriguez, E. R. et al. (2024) Protein Science 33.
- Petersson, M. et al. (2025) Nature Communications 16.
- Xu et al. (2025) Journal of Animal Science and Biotechnology 16:78.


.png)