Allergenicity, Genotoxicity and Subchronic Toxicity Assessment of IgG Binding Protein LT Produced From Aspergillus oryzae


Research Summary
Gastrointestinal health is one of the fastest growing areas in the food and beverage industry, as its importance to overall healthand well-being is becoming increasingly recognized. Immunoglobulins play a key role in protecting the gastrointestinal tract,and nonbovine sources of immunoglobulins (including camel milk, which has a long history of consumption in East Africa andAsia) are increasing in popularity in Western countries as functional foods, particularly for individuals with allergies or intolerances to cow's milk. The physiological benefits of consuming certain heavy-chain immunoglobulins from camel milk relateto the binding domains of camelid single-domain antibodies; thus, a novel binding protein termed “immunoglobulin G (IgG)binding protein LT” (a dimer of two camelid single-domain antibody protein sequences) has been developed for use in food andbeverage products, to provide some of the physiological benefits attributed to consuming camel milk, on an industrial scale. Tosupport the safety of IgG binding protein LT for such use, a comprehensive safety assessment (in silico allergenicity assessment,in vitro genotoxicity studies [bacterial reverse mutation test and in vitro mammalian cell micronucleus test], and a 90-day gavagetoxicity study in rats) was conducted. The in silico allergenicity assessment results demonstrate that IgG binding protein LT ishighly unlikely to pose a risk of allergenic cross-reactivity, and there was no evidence of genotoxicity in vitro. There were no testarticle–related effects in the 90-day toxicity study. These data demonstrate the safety of IgG binding protein LT for its intendeduses in foods and beverages.
1 Introduction
Functional food and beverage products are becoming more popular, as consumers become more health conscious and increasingly seek food or supplement products that provide health benefits in addition to basic nutrition. The relevance of the diet to human health has been long recognized, and preventative approaches are trending within healthcare, where there is a shift from reactive to proactive approaches (Grand View Research 2022).
One of the fastest growing areas of the food and consumer health industry is gastrointestinal health. Fiber-rich foods and fermented dairy products have conventionally been recommended to support gastrointestinal health; however, in more recent years, foods, beverages, and supplements containing ingredients with reported health benefits—such as probiotics, prebiotics, and "synbiotics" (mixtures of probiotics and prebiotics)—have been populating the market, correlating with a growing number of registered human intervention studies substantiating the safety and efficacy of these ingredients (Davani-Davari et al. 2019; Dronkers et al. 2020).
The most investigated and acknowledged probiotics are the bacteria Lactobacillus rhamnosus GG (LGG) and Bifidobacterium animalis subsp. lactis BB12, as well as the yeast Saccharomyces cerevisiae boulardii, all of which are used against different gastrointestinal conditions (Damián et al. 2022; Sanders et al. 2019). While these live probiotics are among the most frequently formulated into various food and supplement products, they can present challenges related to processing and shelf-life stability of the final products; because water activity and storage temperature are the main factors that impact stability, they need to be carefully controlled during and after manufacturing to ensure that the final product meets the probiotic count (colony-forming units) stated on the label (Fenster et al. 2019).
In the category of prebiotics—defined as substrates that are selectively utilized by host microorganisms conferring a health benefit (Gibson et al. 2017)—galacto-oligosaccharides (GOS) and fructo-oligosaccharides (FOS) have perhaps been the most broadly tested and applied by the industry over the last few decades (Davani-Davari et al. 2019). As their natural presence in foods is rather low (too low for an actual health benefit), they are manufactured industrially by enzymatic synthesis, or microbial fermentation, and added to a diverse range of foods at higher concentrations than would occur naturally (Ferreira et al. 2023). More recently, microbially produced human milk oligosaccharides (HMOs) have been successfully upscaled and commercialized (Bych et al. 2019). HMOs are associated with health benefits in infants, and their oral supplementation has been subject to extensive clinical research in both infants and adults (Schönknecht et al. 2023). As oligosaccharides, these prebiotics do not face the same stability issues as live probiotics, which has allowed them to be widely used to create infant formulas that more closely reflect the composition of human breastmilk; however, due to the relatively large daily intake required to achieve a beneficial effect from supplementation (ranging from 4 to 20g/day in studies conducted to date [Elison et al. 2016; Palsson et al. 2020; Ryan et al. 2021]), the use of HMOs in general foods for health benefits may be limited by their potential to impact the physicochemical, technological, and sensory properties of general foods.
Looking beyond HMOs, the gut health-promoting ability of mother's milk is continuing to serve as inspiration for innovators in the functional ingredient space, and of increasing recent interest are products using immunoglobulins (antibodies). (Hurley and Theil 2011; Morrin et al. 2021). Immunoglobulins (a family of globular proteins with protective bioactivities) are among the most important components of all types of milks and colostrum, including those from humans, cows, goats, sheep, and camels (Farooq et al. 2024; Hurley and Theil 2011; Kessler et al. 2019; Swelum et al. 2021). The immunoglobulins found in milk and colostrum are mainly transferred from the mother's circulation and are key to homologous transfer of passive immunity between mother and offspring. These proteins possess different immunological activities depending on the immune status of the mother, and by binding to different specific pathogens and their metabolites, they can aid in the protection of the recipient infant's gastrointestinal tract (Schroeder and Cavacini 2010).
Numerous studies have shown that the protective nature of immunoglobulins can transfer across species, as frequently demonstrated and reviewed in the case of oral ingestion of bovine immunoglobulins by humans (Ulfman et al. 2018). Although bovine milk contains immunoglobulin concentrations at approximately 0.5-1g/L, it is a questionable source of functional immunoglobulins for large-scale consumption, as this group of proteins is labile at the conventional pasteurization temperatures (approximately 65°C) used during typical dairy processing (Gapper et al. 2007; Hurley and Theil 2011). Furthermore, the titers of relevant specificities may be too low for actual health benefits, even if milder processing conditions are used. Other products that are a naturally good source of, or are enriched with, immunoglobulins include bovine and camel colostrum, hyperimmune milk, immunoglobulin G (IgG)-enriched whey powder, purified bovine serum globulin, and avian egg immunoglobulin Y (IgY). These protein preparations have all been upscaled industrially and commercialized in foods or supplements, and many of these products have also been evaluated clinically (Gapper et al. 2007; Mehra and Kelly 2006; Petschow et al. 2014; Proliant Health Ingredients Inc. 2008; Ramani et al. 2024; Yakhkeshi et al. 2022).
Of these novel sources of immunoglobulins, there has been increasing interest and popularity in nonbovine sources such as camel milk, which is not only a good source of immunoglobulins but also has a higher content of certain vitamins (particularly C and B3) than bovine milk and is accessible to consumers with bovine milk allergies (because camel milk lacks beta-lactoglobulin), as well as those who are lactose intolerant (Ait El Alla et al. 2023; Breulmann and Böer 2010; Cardoso et al. 2010; Farah 2011; Konuspayeva and Faye 2021; Smits et al. 2011). Camel milk consumption is not a new phenomenon; it has long been a source of food in arid and semiarid environments such as East Africa and parts of Asia, where camel milk is a major contributor to the human diet (Mbogo et al. 2012; Ngeiywa and Njanja 2013; Seifu 2009; Wilson 1998). However, interest in camel milk as functional food, owing to its reported physiological benefits, is now growing in other parts of the world, including Europe and North America (Ait El Alla et al. 2025).
The simpler structure of immunoglobulins (heavy-chain only) from camel milk gives it a unique potential for industrial use compared to its bovine counterpart (Hamers-Casterman et al. 1993). The binding domains of camelid IgG2/3 (also called single-domain antibodies) have received attention due to their functional potential as gut-stabilizing ingredients (Fill et al. 2022; Jenkins et al. 2024; Petersson et al. 2023). These domains are small (12–15 kDa), robust against high temperatures (63°C–100°C) associated with pasteurization, and generally have high solubility, as well as the ability to be expressed in microorganisms and to be manufactured by precision fermentation at low cost (Arbabi-Ghahroudi 2017; Muyldermans 2013; Tripathi and Shrivastava 2019; US FDA 2024). Depending on the exact identity of these binding proteins, they can be highly functional proteins supporting the safe passage of pathogens through the gastrointestinal tract, maintaining gut health just like immunoglobulins in milk. By taking advantage of the characteristics of these binding proteins, the natural properties of milk immunoglobulin enable them to be used as novel human gut-stabilizing ingredients that can be added to foods and beverages, with the overall purpose to reinforce the IgG content naturally present in the diet by adding only a relatively small amount of functional protein (i.e., an amount that is not expected to influence the texture or flavor of the food preparation).
One such binding protein intended for use in food and beverage products is IgG binding protein LT, a dimer of two camelid single-domain protein sequences (previously discovered and expressed in yeast [Harmsen et al. 2009]), which binds and facilitates excretion of an unhealthy metabolite that is secreted by Escherichia coli and is frequently and unintentionally ingested as part of food. IgG binding protein LT is produced by precision fermentation at scale from a bioengineered fungi Aspergillus oryzae, which is a safe host strain lineage known for food enzyme production (Gomi 2014; Tu et al. 2025).
To meet the regulatory safety requirements to permit the use of IgG binding protein LT for use as an ingredient in foods and beverages, a comprehensive safety assessment (comprising an assessment of allergenicity in silico, genotoxicity in vitro, and subchronic toxicity in vivo) was conducted. Because effectively all known food allergens are proteins, it is critical to evaluate the allergenic potential of any novel protein (FAO/WHO 2009a). Therefore, to assess the potential allergenicity of IgG binding protein LT, the stepwise in silico approach developed and standardized by the Food and Agriculture Organization/World Health Organization of the United Nations and the Codex Alimentarius (Codex Alimentarius 2003, 2009; FAO/WHO 2001) was followed. This approach is currently considered to be the default for assessing the potential cross-reactivity of novel proteins and has been endorsed by regulatory authorities such as the European Food Safety Authority (EFSA), who has implemented this approach into guidance on the requirements for assessing allergenicity of genetically modified plants (EFSA GMO Panel 2017) as well
as those for safety assessments of food enzymes (EFSA CEP Panel 2021) and most recently for novel foods (EFSA NDA Panel 2024). To assess the potential genotoxicity and subchronic toxicity of IgG binding protein LT, the well-established tiered approach to toxicity testing recommended by authorities such as the US Food and Drug Administration and EFSA was followed (EFSA ANS Panel 2021; US FDA 2007); therefore, as is required under this approach for food ingredients that are not systemically available, in vitro genotoxicity (bacterial reverse mutation test [Organisation for Economic Co-operation and Development {OECD} Test Guideline {TG} 471—OECD 1997] and in vitro mammalian cell micronucleus test [OECD TG 487—OECD 2016]) and a subchronic toxicity study in rats (OECD TG 408—OECD 2018) were conducted to conclusively demonstrate the safety IgG binding protein LT.
2 Materials and Methods
2.1 In Silico Allergenicity Assessment
An in silico assessment of the sequence homology of the amino acid sequence of IgG binding protein LT was conducted by Intertek Health Sciences Inc. (Mississauga, ON, Canada) to assess potential cross-reactivity with documented allergens, in accordance with the methodology described by FAO/WHO (2001), Codex Alimentarius (2003, 2009), EFSA GMO Panel (2017), and EFSA CEP Panel (2021). In accordance with these guidelines, the following sequence homology searches were conducted with the amino acid sequence of IgG binding protein LT against the AllergenOnline database (Version 22; http://www.allergenonline.org/) as maintained by the Food Allergy and Resource Program of the University of Nebraska-Lincoln:
- a "sliding window" of 80 amino acid sequences (Segments 1–80, 2–81, 3–82, etc.) derived from each full-length amino acid sequence of each protein (search parameters: minimum identity cut-off = 35%);
- an eight–amino acid exact match; and
- the full-length amino acid sequence (search parameters: FASTA36; E-value cut-off = 1 and maximum alignments of 20).
It is noted that FAO/WHO (2001), Codex Alimentarius (2003, 2009), and EFSA GMO Panel (2017) guidelines recommend searches with the 80–amino acid sliding window and eight–amino acid exact match and do not include a recommendation to conduct searches with the full-length sequence. However, it has been reported in the scientific literature that sequence matches with less than 50% identity over the full-length amino acid sequence are unlikely to be crossreactive, and that clinically important immunoglobulin E (IgE) cross-reactivity is common for proteins sharing > 70% identity (Aalberse 2000; Aalberse et al. 2021; Goodman et al. 2008; Scaife et al. 2024). Given that structural sequence homology between folded proteins may be more accurately evaluated using the full-length amino acid sequences, as noted by Aalberse (2000), Goodman et al. (2008), Adelmouled et al. (2021), and Scaife et al. (2024), any matches identified from the 80–amino acid sliding window or the eight–amino acid exact match searches were further evaluated for the degree of significance and identity over the full sequence, and only considered further in the present allergenicity assessment if the match had an E value < 1 × 10⁻⁷ and a percent identity ≥50% over the full sequence. Any matches sharing > 50% identity over the full amino acid sequence would be further investigated for the clinical relevance of the source, including an investigation of the source of the matched allergen (i.e., whether it is a major food allergen in the United States or Europe) and the relevant route(s) of exposure to which the allergen is reported to elicit allergic responses.
In addition to the sequence homology searches against the AllergenOnline database, further allergenic protein predictions were conducted using the Allermatch database (http://allermatch.org/), which is maintained by Wageningen University and is composed of known allergenic proteins from the UniProtKB allergen database, the World Health Organization/International Union of Immunological Societies (WHO/IUIS) list of allergen nomenclature, and the COMPARE database. Searches of the Allermatch database were conducted using default search parameters with the FASTA algorithm and included the full sequence search, the sliding 80–amino acid window search, and exact match of six and eight amino acids, using the same search criteria as described for the AllergenOnline searches above.
The AllerCatPro 2.0 program was also used to evaluate the potential cross-reactivity of IgG binding protein LT (Nguyen et al. 2022). The AllerCatPro 2.0 search tool includes both primary sequence alignment searches and 3D structure comparisons of a query protein against a database comprised of 9775 allergenic proteins, 162 low allergenic proteins, and 165 autoimmune allergens from the WHO/IUIS, COMPARE, AllergenOnline, UniProtKB/Swiss-Prot, and Allergome databases (Nguyen et al. 2022). The AllerCatPro 2.0 algorithm incorporates the weight of evidence (gluten-like Q-repeats, 3D epitopes, linear-window rule, and hexamer hits) and reports the level of evidence related to the potential allergenicity of the query protein (i.e., no evidence, weak evidence, or strong evidence). Nguyen et al. (2022) reported that using a test dataset of 218 positive and 212 negative allergens, AllerCatPro 2.0 achieved 84.7% accuracy with 100% sensitivity and 68.9% specificity, and that using a larger dataset (2015 positive and 2015 negative allergens), similarly favorable performance outcomes were obtained (96.0% accuracy with 93.2% sensitivity and 98.8% specificity).
2.2 Toxicity Studies
2.2.1 Regulatory Compliance
The toxicity studies were conducted at Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)–accredited facilities, and, with the exception of the 14-day oral toxicity study in rats, which was a preliminary dose range–finding study conducted solely to aid dose selection for the repeated-dose 90-day oral toxicity study in rats, the studies were conducted in compliance with Good Laboratory Practice (following OECD Principles on Good Laboratory Practice [OECD 1998] and the United Kingdom Good Laboratory Practice Regulations 1999 [UK Government 1999]) and following the methods described in OECD TG 471 (OECD 1997), OECD TG 487 (OECD 2016), and OECD TG 408 (OECD 2018) for the bacterial reverse mutation test, in vitro mammalian cell micronucleus test, and repeated-dose 90-day oral toxicity study in rats, respectively.
The in vivo studies were conducted in compliance with the UK Animals (Scientific Procedures) Act 1986 (ASPA) Amendment Regulations 2012 (UK Government 2012), and a local ethical review of the protocols was conducted prior to the studies commencing. The reporting of the in vivo studies in this publication follows the recently updated Animal Research: Reporting of In Vivo Experiments (ARRIVE) Guidelines 2.0 for reporting animal research (Percie du Sert et al. 2020), which represents best practice in the reporting of animal studies.
2.2.2 Test Materials
The test article, IgG binding protein LT, was produced by pure culture submerged fed-batch fermentation of a genetically modified strain of A. oryzae (the production organism is removed from the final ingredient) and was supplied by Bactolift ApS (Copenhagen, Denmark) as a brownish liquid comprising approximately 53% protein (31% pure IgG binding protein LT), 40% carbohydrates, and 3% ash, on a dry weight basis. All concentrations and doses used in the studies were corrected for pure IgG binding protein LT content and are expressed on that basis throughout this publication.
Purified water (Labcorp, Harrogate, UK) was the vehicle for the 14- and 90-day oral toxicity studies. For the genotoxicity studies, sterile water (Labcorp, Huntingdon, UK) was used as the vehicle and negative control. Positive controls (2-nitrofluorene [CAS: 607-57-8; purity: 98%]; 4-nitroquinoline [CAS: 56-57-5; purity: 98%]; 2-aminoanthracene [CAS: 613-13-8; purity: 96%], methyl methanesulfonate [CAS: 66-27-3; purity: 99%], acridine mutagen ICR 191 [CAS: 10070-68-1; purity: 99%], and benzo[a]pyrene [CAS: 50-32-8; purity: 96%] for the bacterial reverse mutation test; mitomycin C [CAS: 50-07-7; purity: 98%], colchicine [CAS: 64-86-8; purity: 99%], and cyclophosphamide [CAS: 50-18-0; purity: 93.7%] for the in vitro mammalian cell micronucleus test) were supplied by Sigma-Aldrich Chemical (St. Louis, MO, USA). The mammalian liver postmitochondrial fraction (S9) used for metabolic activation in the genotoxicity studies was obtained from Molecular Toxicology Inc. (Boone, NC, USA), where it was previously prepared from male Sprague Dawley rats induced with β-naphthoflavone/phenobarbital. S9 batches had been previously checked by the manufacturer for sterility, protein content, ability to convert ethidium bromide and CPA to bacterial mutagens, and cytochrome P450–catalyzed enzyme activities (alkoxyresorufin-O-dealkylase activities). The bacterial strains used in the bacterial reverse mutation test (mutant histidine-dependent Salmonella enterica serovar Typhimurium strains TA98, TA100, TA1535, and TA1537 and Escherichia coli tryptophan-dependent strain WP2 uvrA pKM101) were also sourced from Molecular Toxicology Inc. (Boone, NC, USA). The human peripheral blood lymphocytes for the in vitro mammalian cell micronucleus test were obtained from blood collected from healthy nonsmoking adult donors aged 20–31 years (two donors per experiment).
2.2.3 Genotoxicity Studies
2.2.3.1 Bacterial Reverse Mutation Test (Ames Test)
Two separate experiments were performed using the "treat and wash" procedure, which is a commonly recommended method when the test article contains amino acids (such as proteins) that may be released and cause artefactual growth stimulation in a standard plate-incorporation test (Thompson et al. 2005); the "treat and wash" procedure is routinely recommended by EFSA for the testing of novel proteins and food enzymes (which are also proteins) in the bacterial reverse mutation test (EFSA CEP Panel 2021; EFSA NDA Panel 2024).
The bacterial strains were exposed to IgG binding protein LT at concentrations of 5, 16, 50, 160, 500, 1600, or 5000 μg/mL in the first experiment (the first experiment was repeated with strain TA98 in the presence of S9 using these same concentrations, to elucidate whether an increase in revertant colony numbers was biologically relevant) and at 50, 160, 500, 1600, or 5000 μg/mL in the second experiment, in the presence and absence of S9 metabolic activation. Concurrent negative control and positive control (2-NF [strain TA98], 4-NQO [strains TA100 and WP2 uvrA pKM101], MMS [strain TA1535], and ICR 191 [strain TA1537] in the absence of S9; B[a]P [strain TA98] and AAN [strains TA100, TA1535, TA1537, and WP2 uvrA pKM101] in the presence of S9) were included under all conditions.
To prepare the bacterial suspensions, tubes of bacterial culture were first centrifuged at 2000g for 10 min to remove the culture medium. The resultant bacterial pellets were resuspended in 100-mM phosphate buffer (pH 7.4) to provide a bacterial suspension concentration of approximately 10⁹ cells/mL. Either IgG binding protein LT, the vehicle, or positive control (0.1 mL of each) was added to 1-mL aliquots of the bacterial suspension, before addition of 0.44 mL of S9 (10% in the first experiment, 20% in the second experiment—for treatments with metabolic activation), or buffer solution (for treatments without metabolic activation). All tubes were then incubated at 34°C–39°C, with shaking, for 90 min.
After incubation, for the "treat and wash" part of the assay, 12 mL of a wash solution (nutrient broth in pH 7.4 phosphate-buffered saline 1:7 [v/v]) was added to each culture, and the washed bacteria were collected by centrifugation at 2000g for 30 min. The supernatant was discarded, leaving approximately 1 mL of bacteria resuspended in the residual supernatant; the wash procedure was then repeated. To determine revertant colony numbers, a total of 0.1 mL of each bacterial suspension was then added to duplicate to 2-mL molten supplement top agar and mixed and poured onto the surface of Vogel-Bonner E agar plates (Southern Group Laboratory, Corby, UK; E&O Laboratories Ltd., Bonnybridge, UK; Molecular Toxicology Inc., Boone). Bacterial suspensions were also diluted with isotonic saline for approximately 10⁸ cells/mL, and 0.1-mL aliquots were added, in triplicate, to 2 mL of molten top agar (Oxoid, Basingstoke, UK), mixed, and then poured onto nutrient agar plates (Oxoid) for assessment of viability. Plates were prepared without the addition of bacteria to assess sterility of IgG binding protein LT, S9, and sodium phosphate buffer.
Once set, the plates were inverted and incubated (in the dark) for either 1 (plates for viability assessment) or 3 days (plates
for mutagenicity assessment) at 34°C–39°C. Following incubation, the plates for the mutagenicity assessment were examined for evidence of toxicity to the background lawn, and revertant colonies were counted electronically using a Sorcerer Colony Counter (Perceptive Instruments, Bury St Edmunds, UK); viability counts were also scored electronically at the end of the incubation period.
The assay was considered valid if negative control counts were consistent with the laboratory's historical negative control database and if the concurrent positive controls induced increases in mean revertant colony numbers of at least 2-fold (strains TA98, TA100, and WP2 uvrA pKM101) or 3-fold (strains TA1535 and TA1537), compared with the negative control. A positive mutagenic response was defined as a concentration-related and/or reproducible ≥2-fold (strains TA98, TA100, and WP2 uvrA pKM101) or ≥3-fold (strains TA1535 and TA1537) increase in mean revertant colonies, compared with the negative control.
2.2.3.2 In Vitro Mammalian Cell Micronucleus Test
The potential clastogenicity and aneugenicity of IgG binding protein LT was assessed using previously described methods (Aardema et al. 1998; Elhajouli et al. 1998; Fenech 1998; Fenech et al. 1999; Fenech et al. 2003; Migliore and Nieri 1991; Miller et al. 1998; Rosefort et al. 2004; Thybaud et al. 2007).
Whole blood cultures from donated blood were established by adding pooled blood and phytohaemagglutinin (PHA) to Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich); the cultures were then incubated at 34°C–39°C, and the cells were resuspended (twice daily) by gentle inversion. Short-term (3-h exposure followed by 17-h recovery) and continuous (20-h exposure, without recovery) treatments were conducted 44–48 h following the mitogen stimulation by PHA. S9 mix (10%) or 150-mM potassium chloride (KCl) (for short-term treatments with or without metabolic activation, respectively) or 150-mM KCl and cytochalasin B (Sigma-Aldrich) (for continuous treatment without metabolic activation) were added to each of the cultures, before incubation at 34°C–39°C for the appropriate length of time (i.e., 3 h for short-term treatment; 20 h for continuous treatment).
To aid in the selection of concentrations for the main micronucleus experiment, a cytotoxicity range-finder experiment was carried out in which the human lymphocyte cultures were exposed to the negative control (sterile water) or IgG binding protein LT (at 9.8, 19.5, 39.1, 78.1, 156.3, 312.5, 625, 1250, 2500, or 5000 μg/mL), with 5000 μg/mL being the recommended maximum concentration where the test article is not of defined composition [e.g., complex mixtures] or is a biological material [OECD 2016]) under short-term and continuous conditions. The main micronucleus experiment was conducted in the same manner as the cytotoxicity range finder, except positive controls (MMC and COL [short-term and continuous treatments in the absence of S9] and CPA [short-term treatment in the presence of S9]) were also included, and different concentrations were selected for the main experiment based on cytotoxicity (assessed using the replication index [RI]) observed in the range-finder experiment. In the short-term treatments, none of the concentrations induced cytotoxicity above the maximum of 50%–60% recommended in OECD TG 487 (OECD 2016); therefore, four concentrations up to 5000 μg/mL were tested under these conditions, with the highest three (3000, 4000, and 5000 μg/mL) selected for micronucleus analysis. For the continuous treatment, an RI above 60% was observed at three of the concentrations tested in the range-finder experiment (data not shown); therefore, in accordance with OECD TG 487 (OECD 2016), a range of closely spaced concentrations (14, ranging from 150 to 5000 μg/mL) were tested in the main experiment, with three of those (150, 1500, and 2321.34 μg/mL, because 2321.34 μg/mL induced an acceptable top concentration RI of 54%) selected for micronucleus analysis. In the main experiment, duplicate cultures were prepared for each IgG binding protein LT concentration and positive control, and quadruplicate cultures were prepared for the negative control.
At the end of each treatment, at the defined sampling time, cultures were centrifuged at 500g for 5 min, before the supernatant was discarded and the remaining cells were resuspended in KCl at 34°C–39°C for 3 min, to allow cell swelling. The cultures were then agitated, and the cells fixed by adding cold 3:1 (v/v) methanol/glacial acetic acid (Fisher Scientific, Loughborough, UK) onto the culture surface and then slowly inverting to mix. After two additional centrifugation occasions (approximately 500g for 5 min), removal of the supernatant, and resuspension of the cell pellet, additional fresh fixative was added, and the cells were stored refrigerated (2°C–8°C) for a minimum of 30 min. To prepare the slides for analysis, 50 μL of cell suspension was spread onto microscope slides (Leica, Deerfield, IL, USA), which were then air-dried at room temperature, before staining by immersion in 12.5-μg/mL acridine orange (Sigma-Aldrich, USA) and then in water. The stained slides were air-dried and stored at room temperature, in the dark, until analysis. Immediately before analysis, one to two drops of purified water were added to the slides before mounting with glass coverslips, and scoring was carried out using a Nikon Laboratory G fluorescence microscope (Nikon, Tokyo, Japan). A minimum of 1000 binucleate cells from each culture (2000 per IgG binding protein LT concentration and positive control; 4000 for the negative control) were analyzed for micronuclei.
The assay was considered valid if all the following criteria were met: (1) the binomial dispersion test demonstrated acceptable heterogeneity (in terms of micronucleated binucleate cell frequency) between replicate cultures; (2) the frequency of micronucleated binucleate cells in vehicle controls fell within the historical negative control 95th percentile ranges; (3) the positive controls induced responses that were compatible with the laboratory's historical positive control database and produced statistically significant increases compared with the concurrent negative control; (4) micronucleated binucleate cell frequencies in both replicate cultures for at least one positive control concentration analyzed were above the 95th percentile of the respective historical negative control range; (5) a minimum of 50% of cells had gone through at least one cell division (as measured by binucleate + multinucleate cell counts) in negative control cultures at the time of harvest; (6) the maximum concentration analyzed under each treatment condition was met the top concentration requirements laid out in OECD TG 487 (OECD 2016); and (7) adequate number of cells (at least 500 per culture) and concentrations (three) were analyzable.
IgG binding protein LT would be considered as clastogenic or aneugenic if it induced a concentration-related (as indicated by a statistically significant trend test) statistically significant increase in the frequency of micronucleated binucleate cells at one or more concentrations, at a frequency that exceeded the 95th percentile of the respective historical negative control range.
2.2.4 Repeated-Dose Toxicity Studies
2.2.4.1 Repeated-Dose 14-Day Oral Gavage Study in Rats
2.2.4.1.1 Animals, Environmental Conditions, and Dosing Schedule
Wistar Han (Crl:WI[Han]) rats (23 of each sex), supplied by Charles River (Margate, UK), were clinically examined on arrival, before 20 of each sex were allocated for use on the study (remaining animals were retained as spares). Each animal was uniquely identified using a subcutaneous electronic transponder. The animals were acclimatized to the test facility for 14 days before dosing, and, on the first day of dosing (when the animals were approximately 6 weeks of age), body weights ranged from 160 to 210 g for males and 128 to 166 g for females. Animals were group housed (up to three of each sex per cage) in polycarbonate cages (Arrowmight, Hereford, UK) that conformed to the Code of Practice for the Housing and Care of Animals Bred, Supplied or Used for Scientific Purposes (UK Government 2014). Wood bedding (Eco Pure Flakes, 5, Datesand, Bredbury, UK) and environmental enrichment (wooden chew blocks and amber tunnels [Datesand]) were provided in each cage. Environmental controls were set to maintain temperature (19°C–25°C) and relative humidity (40%–70%), as well as providing 15 or greater air changes per hour, and a 12:12-h light/dark cycle. Main supply water and 5LF2 EU Rodent Diet (International Product Supplies Ltd., London, UK) were available ad libitum (except for an overnight fast before collection of blood samples for hematology and clinical chemistry on Day 12 of dosing and for coagulation on the day of necropsy).
Using a total randomization procedure, animals were assigned to one of four dose groups (five animals of each sex per group) receiving either the vehicle control (purified water) or IgG binding protein LT (formulated as a solution in the vehicle) at 105, 350, or 1050 mg/kg body weight/day, by gavage, for 14 consecutive days, until the day before necropsy. As specific exposure estimates and preliminary toxicity data were not available when the study was conducted, the high dose of 1050-mg/kg body weight/day was selected, as it is approximately equivalent to the 1000 mg/kg body weight/day recommended high dose for a limit test in OECD TG 408 (OECD 2018); approximately 3-fold descending intervals were used for the mid and low doses. A constant dose volume of 20 mL/kg (the highest recommended in OECD TG 408 for test articles administered as aqueous solutions) was used.
2.2.4.1.2 Clinical Observations
Cage-side observations for signs of ill health or overt toxicity were conducted twice per day (once on the days of arrival and necropsy), with more detailed individual observations (outside the cage) of each animal conducted once during the acclimatization period, once on the first day of dosing, and once per week thereafter. Observations for clinical signs associated with dosing were conducted for each animal approximately 30 min and 1, 2, and 4 h after administration of each dose.
2.2.4.1.3 Body Weight and Food Consumption
Body weights were recorded once on Day 8 of the acclimatization period, once just before administration of the first dose, and twice weekly thereafter, up to and including the day of necropsy. The amount of food consumed for each cage was recorded twice per week and was calculated and reported as grams consumed per animal per day (i.e., the total amount consumed for each cage was divided by the number of animals in each cage for the reporting of food consumption).
2.2.4.1.4 Clinical Pathology
Following an overnight fast, blood samples were collected from all animals on Day 12 of dosing (for analysis of hematology and clinical chemistry parameters) and terminally at necropsy (for analysis of coagulation parameters). Blood samples for analysis of hematology (red blood cell [RBC] count, hemoglobin, hematocrit, mean corpuscular volume [MCV], mean corpuscular hemoglobin [MCH], mean corpuscular hemoglobin concentration [MCHC], red cell distribution width [RDW], reticulocyte count, platelet count, white blood cell [WBC] count, neutrophils, lymphocytes, monocytes, eosinophils, basophils, and large unstained cells [LUC]) and coagulation (prothrombin time [PT], activated partial thromboplastin time [APTT], and fibrinogen) parameters were collected into tubes containing trisodium citrate (Starlab, Blakelands, UK) or potassium ethylenediaminetetraacetic acid (EDTA) (Starlab) anticoagulant, respectively. Samples for clinical chemistry (glucose, blood urea nitrogen [BUN], creatinine, total protein, albumin, globulin, albumin:globulin ratio, total cholesterol, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol, triglycerides, total bilirubin, alanine aminotransferase [ALT], alkaline phosphatase [ALP], aspartate aminotransferase [AST], gamma-glutamyl transferase [GGT], creatine kinase, calcium, inorganic phosphorus, sodium, potassium, and chloride) tests were collected into serum separator tubes (Starlab) without anticoagulant. Tubes containing the blood samples were fully inverted approximately 10 times, followed by (for hematology and coagulation samples only) mixing for at least 5 min on an automated mixer (Thermo Scientific, UK); samples for clinical chemistry were not mixed further, instead being left to clot before analysis.
2.2.4.1.5 Organ Weights and Macroscopic Examinations
On the day after receiving their final dose, all animals were weighed and anesthetized with isoflurane, before being exsanguinated and subjected to gross macroscopic necropsy, where an examination of the external features of the carcass; external body orifices; abdominal, thoracic, cranial, pelvic, and oral cavities; organs; and tissues was conducted.
The following organs and tissues were collected from each animal and weighed, following dissection of fat and other contiguous tissues: adrenals (weighed together), aorta, femur with bone marrow (including the femorotibial joint), sternum with bone marrow, brain, cecum, coagulating gland, colon, duodenum, epididymides (weighed together), esophagus, eyes, gut-associated lymphoid tissue (Peyer's patch), Harderian glands, heart, ileum, jejunum, kidneys (weighed together), liver, lung with large bronchi, mandibular and mesenteric lymph nodes, mammary
gland, bicep, sciatic nerve, optic nerves, ovaries (with oviducts), pancreas, pituitary gland, prostate gland, rectum, mammary salivary gland, seminal vesicles, skin/subcutis, spinal cord (cervical, lumbar, and thoracic), spleen, stomach, testes (weighed together), thymus, thyroid (with parathyroid), tongue, trachea, urinary bladder, uterus (with cervix), and vagina.
2.2.4.2 Repeated-Dose 90-Day Oral Gavage Study in Rats
2.2.4.2.1 Animals, Environmental Conditions, and Dosing Schedule
A total of 53 male and 53 female Wistar Han (Crl:WI[Han]) rats were supplied by Charles River Laboratories (Margate, UK), and after all were clinically examined on arrival, 50 of each sex were allocated for use on the study (remaining animals were retained as spares). Each animal was uniquely identified using a subcutaneous electronic transponder (tail marking was also used to identify animals during functional observational battery tests). The animals were acclimatized to the test facility for 15 days before dosing, and on the first day of dosing (when the animals were approximately 7 weeks of age), body weights ranged from 145 to 203 g for males and 110 to 157 g for females. The housing conditions (except for the wood bedding, which was provided by a different supplier [Datesand, UK]), environmental enrichment, and environmental controls were the same as described for the 14-day study (see Section 2.2.4.1.1).
Using the same randomization procedure as described for the 14-day study (see Section 2.2.4.1.1), animals were assigned to one of four dose groups (15 animals of each sex in the vehicle control and high-dose groups; 10 animals of sech sex in the low- and mid-dose groups) and administered either the vehicle control (purified water) or IgG binding protein LT (formulated as a solution in the vehicle) at 50-, 150-, or 450-mg/kg body weight/day, once daily, by gavage, for at least 90 consecutive days, until the day before necropsy (10 animals of each sex) or before starting a 4-week off-dose recovery period (five animals of each sex in the vehicle control and high-dose groups). Although the 20-mL/kg dose volume used in the 14-day study appeared to be well tolerated in that study, it is widely accepted that gavage administration volumes should be kept as low as practicably possible, to reduce the potential for causing stress to the animals (Brown et al. 2000; Turner et al. 2011). Therefore, it was decided to use a dose volume of 10 mL/kg for this longer term 90-day study, which still allowed for selection of a high dose (450-mg/kg body weight/day) that, on the basis of internal exposure estimates (which had been conducted shortly before the start of this study), would provide a more than sufficient margin of exposure compared with highest estimated intakes from the use of IgG binding protein LT in foods (see Section 4). Descending 3-fold intervals were used to select the mid and low doses.
2.2.4.2.2 Clinical Observations
Cage-side observations (for signs of ill health or overt toxicity) were conducted twice daily throughout the study and once on the days of arrival and necropsy, with more detailed individual observations (observing potential effects on behavior, gait, posture, respiration, discharge, excretion, involuntary movements, skin, tail, eyes, pelage, and activity) conducted once during the acclimatization period and once weekly thereafter. Postdose observations (2 h after dosing) were conducted once daily during Week 1, twice weekly from Weeks 2 to 4, and once weekly thereafter. Eyes were examined using an indirect ophthalmoscope and slit-lamp biomicroscope (SJ Hales Ltd., UK) on Day 11 of the acclimatization period (all animals) and on Day 78 of dosing (control and high-dose groups only). A functional observational battery test (where animals were observed for the same parameters as in the weekly detailed examinations, as well as for their reaction to auditory, visual, and proprioceptive stimuli, and quantitative measurements of latency to first step, number of rears, fecal boli, urine pools, forelimb and hindlimb grip strength, hindlimb foot splay, and body temperature) was conducted on Day 9 of acclimatization, Day 90 of dosing, and Day 28 of recovery. Locomotor activity (basic and fine movements, total ambulation, total distance traveled, and number of rearing events) of all surviving animals was recorded using the Kinder Motor Monitor System (Kinder Scientific, Chula Vista, CA, USA) on Day 14 of the acclimatization period and at the end of the dosing and recovery periods.
2.2.4.2.3 Body Weight and Food Consumption
Body weights were recorded once on Day 9 of the acclimatization period, once just before administration of the first dose, and once each week thereafter throughout the dosing and recovery phases, up to and including the day of scheduled necropsy. The food consumption schedule was the same as described for the 14-day study (see Section 2.2.4.1.3).
2.2.4.2.4 Clinical Pathology
Blood samples for hematology, coagulation, and clinical chemistry tests were collected from all surviving animals at the end of the dosing period (Day 92 or 93 of dosing) and at the end of the recovery period (Day 29 of recovery), after an overnight fast, into the same tubes (with or without anticoagulant) and for analysis of the same parameters (except that analysis of total bile acids was also included in the clinical chemistry tests battery) as described for the 14-day study (see Section 2.2.4.1.4). Terminal blood samples were also collected at scheduled necropsy at the end of the dosing and recovery periods for analysis of triiodothyronine (T3), thyroxine (T4), and thyroid-stimulating hormone (TSH). The samples for thyroid hormone analysis were collected at a comparable time of day (between 09:00 and 13:00, to limit the potential confounding effect of natural diurnal variations in circulating thyroid hormone concentrations) into serum separator tubes with clotting activator (Starlab) and were inverted approximately 10 times, before being allowed to clot at room temperature for 30 min, and then centrifuged at 2000g for 10 min at 4°C. Resultant serum was added to amber polypropylene tubes (Starlab) for analysis using liquid chromatography–tandem mass spectrometry (Sciex, Macclesfield, UK) for T3 and T4 analyses or a MILLIPLEX Luminex MAP assay (Merck-Millipore, Watford, UK) for analysis of TSH.
Urine samples were collected from all surviving animals placed in urine collection cages (with water diverters to prevent water contamination) (Arrowmight) on Day 9 of acclimatization and Day 28 or 80 of dosing, and Day 28 or 29 of recovery. Food was removed during urine collection. Urine samples were analyzed for appearance (clarity and color), volume, specific gravity, total protein, glucose, ketones, bilirubin, blood, and microscopic examination of sediment.
2.2.4.2.5 Sperm Analysis and Estrous Cycles
Wet vaginal smears, collected by pipette lavage to assess the stage of estrous, were collected from all female animals for at least 14 days before necropsy, on the day of necropsy, and for females assigned to the recovery period, from Day 15 to 29 of the recovery phase.
For male animals that survived to scheduled necropsy, cauda epididymal sperm number in grams, total sperm per cauda, and vas deferens sperm motility and velocity were recorded by computer-assisted sperm analysis (CASA) using the ToxIVOS system (Hamilton Thorne, Beverly, MA, USA) from a sample of sperm from one epididymis (containing vas deferens and cauda epididymis) of each male. A sperm sample from the vas deferens/epididymal junction of males in the control and high-dose groups was prepared and microscopically evaluated for any abnormalities in sperm morphology (as there were no IgG binding protein LT–related findings in the high-dose group, samples from low- and mid-dose animals were not analyzed).
2.2.4.2.6 Organ Weights and Macroscopic and Microscopic Examinations
At the end of the dosing (Day 92 or 93) and recovery (Day 29) periods, all animals were weighed, anesthetized with isoflurane, exsanguinated, and subjected to gross macroscopic necropsy, where a full list of organs was weighed (as described for the 14-day study, see Section 2.2.4.1.5). Bone marrow smears were prepared from the femur of each animal but were not analyzed, in the absence of any hematological reasons for doing so. For early decedents, and all animals in the control and high-dose groups (at the end of the dosing and recovery periods), the following organs and tissues were preserved in 10% buffered formalin (except for the eyes [Davidson's fixative] and the epididymis and testis [modified Davidson's fixative]), embedded in paraffin, sectioned, and stained with hematoxylin and eosin, before being microscopically examined: adrenals, aorta, brain, cecum, cervix, coagulating glands, colon, duodenum, epididymis, esophagus, eyes, gross lesions, Peyer's patch, Harderian gland, heart, ileum, jejunum, kidneys, liver, lung (with large bronchi), lymph nodes (mandibular and mesenteric), mammary glands, muscle (biceps femoris), nasal cavity, nasopharynx, optic nerve, ovaries, oviducts, pancreas, pituitary gland, prostate, rectum, salivary gland (mandibular), seminal vesicles, skin, spinal cord (cervical, lumbar, and thoracic), spleen, stomach, testis, thymus, thyroid (with parathyroid), tongue, trachea, urinary bladder, uterus, and vagina. For low- and mid-dose animals, slides were prepared for all organs and tissues, but only those with macroscopic changes were examined (as there were no IgG binding protein LT–related findings in the high-dose group).
2.2.5 Statistical Analysis
In the in vitro mammalian cell micronucleus test, the proportions of micronucleated binucleate cells in each replicate were used to establish acceptable homogeneity between replicates with the use of a binomial dispersion test (Richardson et al. 1989), and the proportion of micronucleated binucleate cells for each IgG binding protein LT concentration was compared with the proportion in negative control cultures using Fisher's exact test (Richardson et al. 1989). A Cochran–Armitage trend test was used to determine whether a concentration-related response was evident.
For the in vivo studies, data for each sex were analyzed separately, and only data collected on or after the first day of dosing were statistically analyzed. Analysis of variance (ANOVA) and pairwise (vehicle control versus low-, mid-, and high-dose IgG binding protein LT groups) comparisons were used to statistically analyze body weight (absolute and terminal), body weight change, food consumption, continuous hematology, clinical chemistry and urinalysis values, organ weights (absolute, as well as relative to body weight and brain weight), functional observational battery performance (90-day study only), and thyroid hormones (90-day study only). Levene's test was used before performing the ANOVA, to test for equality of variances between groups, and in the case of a statistically significant (p ≤ 0.05) result, a rank transformation was applied before conducting the ANOVA. Where Levene's test was not statistically significant, an ANOVA was conducted. If the ANOVA results were statistically significant, Dunnett's test was used for pairwise (vehicle control versus low-, mid-, and high-dose IgG binding protein LT groups) comparisons. For comparisons involving only two groups (e.g., during the recovery period of the 90-day study), a two-sample t test was conducted. Where only two groups were available for analysis, a Wilcoxon rank sum test was performed. Motor activity (basic movements, fine movements, total ambulations, total distance traveled, and total rears) were analyzed using a repeated measures ANOVA as has been described previously (Winer 1971). The variance–covariance structure used in the analysis was the autoregressive moving average (1, 1). t tests were used for pairwise (vehicle control versus low-, mid-, and high-dose IgG binding protein LT groups) comparisons.
All statistical tests were evaluated at the 5.0% probability level.
3 Results
3.1 In Silico Allergenicity
The results of the in silico allergenicity searches for IgG binding protein LT are provided in Table 1. Searches using the full-length alignment, 80–amino acid sliding window alignment, and eight–amino acid exact match approaches did not identify any matches with putative allergens from the AllergenOnline and Allermatch databases. In addition, using the AllerCatPro 2.0 search tool, a result of "no evidence" of allergenic potential was achieved for IgG binding protein LT. The results demonstrate that IgG binding protein LT is highly unlikely to pose a risk of allergenic cross-reactivity.
In the 90-day study, estrous cycle regularity was analyzed in Statistical Analysis System (SAS) using Fisher's exact tests (onesided for an increasing response in irregular cycles with IgG binding protein LT groups versus the vehicle control group). Pristima software was used to analyze the mean number of estrous cycles, mean cycle length, and male reproductive parameters (sperm motility, sperm density, and total sperm count), with a Kruskal–Wallis nonparametric ANOVA conducted first. If the Kruskal–Wallis test was statistically significant (p ≤ 0.05), pairwise (vehicle control versus low-, mid-, and high-dose IgG binding protein LT groups) comparisons were made using the Wilcoxon rank sum test. If the Kruskal–Wallis test was not statistically significant (p > 0.05), no further analyses were conducted.
3.2 Genotoxicity Studies
3.2.1 Bacterial Reverse Mutation Test (Ames Test)
Negative control counts were consistent with the laboratory's historical negative control database, and the consistent positive controls induced increases in mean revertant colony numbers of at least 2-fold (strains TA98, TA100, and WP2 uvrA pKM101) or 3-fold (strains TA1535 and TA1537), compared with the negative control, which demonstrates the validity of the assay.

Toxicity (a slight thinning of the background lawn) was observed in the absence of metabolic activation in the first experiment (at 5000 μg/mL for strains TA100, TA1537, and WP2 uvrA pKM101) and in the second experiment (at 1600 or 5000 μg/mL for strains TA1535 and TA1537, and at 5000 μg/mL for strains TA98, TA100, and WP2 uvrA pKM101); no evidence of toxicity was observed in the presence of metabolic activation for any strain. There were no IgG binding protein LT–related differences in the mean numbers of revertant colonies compared with negative controls in either experiment, at any concentration tested, with or without metabolic activation (Table 2). In the first experiment for strain TA98, in the absence of S9, marginally greater than 2-fold increases in mean revertant colony numbers were observed at the lowest (5 μg/mL) and third-lowest (50 μg/mL) concentrations (2.2- and 2.3-fold increases, respectively); however, this was clearly unrelated to administration of IgG binding protein LT, as there was no concentration-related response (mean revertant colony numbers were comparable with controls at the highest IgG binding protein LT concentrations), and it was not reproducible (these differences were not observed in the repeat experiment). IgG binding protein LT was, therefore, clearly negative (nonmutagenic) in this valid bacterial reverse mutation test.
3.2.2 In Vitro Mammalian Cell Micronucleus Test
All criteria for a valid study were met. The binomial dispersion test demonstrated acceptable heterogeneity between replicate cultures; mean frequencies of micronucleated binucleate cells in negative and positive controls fell within the historical negative and positive control 95th percentile ranges, respectively; positive controls induced statistically significant increases in the frequencies of micronucleated binucleate cells compared with the concurrent negative control, with micronucleated binucleate cell frequency for at least one positive control concentration per treatment condition exceeding the 95th percentile of the historical negative control ranges; a minimum of 50% of cells went through at least one cell division in negative control cultures at the time of harvest; the top concentration analyzed under each treatment condition was selected in accordance with the criteria outlined in OECD TG 487 (OECD 2016) (see Section 2.2.3.1); and adequate numbers of cells and concentrations were analyzed.
Exposure of human lymphocyte cultures to IgG binding protein LT in the absence and presence of S9 metabolic activation resulted in mean frequencies of micronucleated binucleate cells that were similar to, and not statistically significantly (p < 0.05) different than, those observed for cultures exposed to the negative control, at all concentrations analyzed, and the mean micronucleated binucleate cell frequencies of all IgG binding protein LT–exposed cultures fell within the historical negative control 95th percentile ranges (Table 3). IgG binding protein LT was, therefore, clearly negative (not clastogenic or aneugenic) in this valid in vitro mammalian cell micronucleus test.
3.3 Repeated-Dose 14-Day Oral Gavage Study in Rats
There were no IgG binding protein LT–related clinical signs or differences in body weight or food consumption between
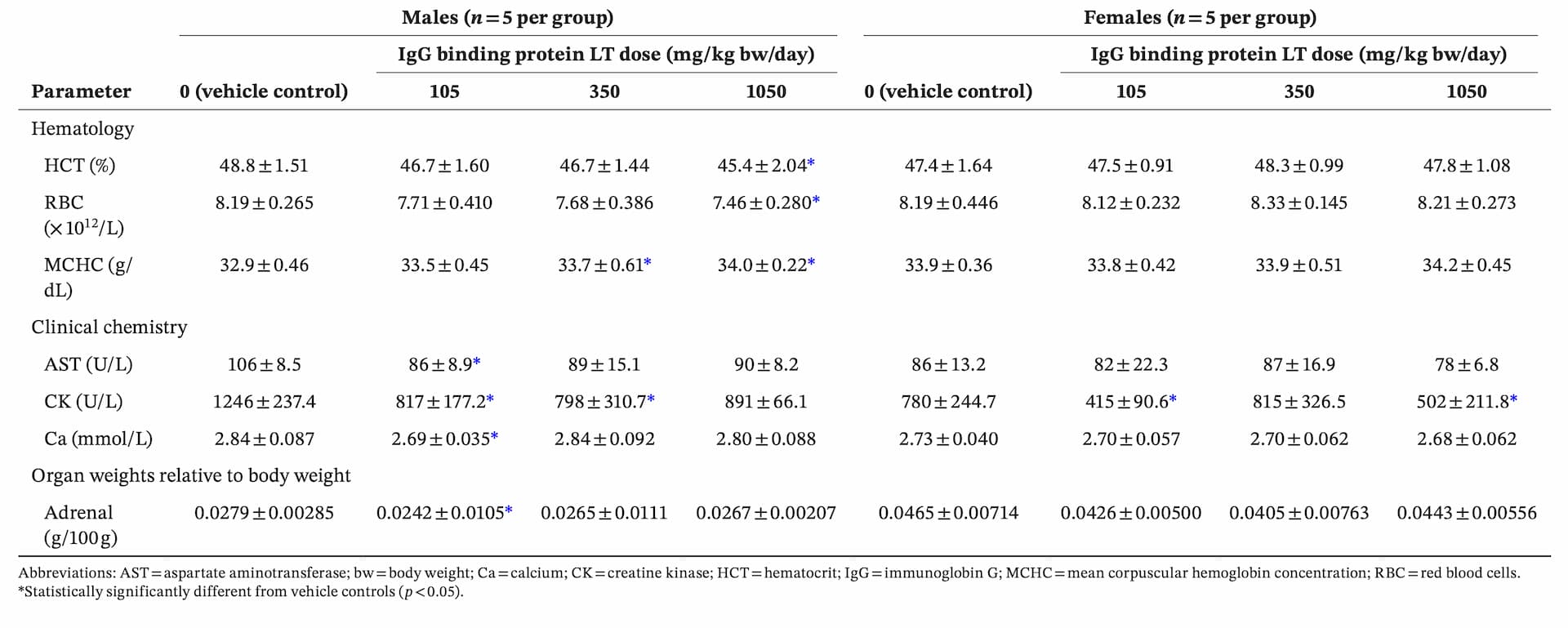
controls and IgG binding protein LT–dosed groups, and there were no IgG binding protein LT–related macroscopic findings at necropsy (data not shown). Where there were statistically significant differences in values for some parameters between IgG binding protein LT groups and controls (Table 4), they were either not dose-related (lower creatine kinase concentration at 105 [both sexes], 350 [males]-, or 1050 [females]-mg/kg body weight/day; lower concentrations of AST and calcium; and lower body weight–relative adrenal weight, for males given 105-mg/kg body weight/day), or on occasions where there was a potential dose–response (lower hematocrit, total RBC, and MCHC for males given 1050-mg/kg body weight/day), all individual values were within historical control range (individual values and historical control data not shown).
3.4 Repeated-Dose 90-Day Oral Gavage Study in Rats
3.4.1 Mortality and Clinical Observations
There were no IgG binding protein LT–related deaths. One male in each of the control and mid-dose groups was found dead on Days 79 and 61 of dosing, respectively; however, neither death was related to IgG binding protein LT administration (for the control male, a large heart was observed macroscopically, with microscopic examination revealing slight multifocal cardiomyocyte degeneration and necrosis, which was considered the cause of death, for the mid-dose male, the cause of death was considered to be severe hydronephrosis of the right kidney) (data not shown). No IgG binding protein LT–related clinical signs were observed during the observations conducted daily (cage-side or postdose observations) or weekly (detailed individual animal examinations), and there were no ophthalmic abnormalities, nor any differences in motor activity or performance in the functional observational battery, that were related to IgG binding protein LT administration (data not shown).
3.4.2 Body Weight and Food Consumption
Mean body weights and food consumption throughout the dosing period are presented in Figure 1 and 2, respectively. The amount of body weight gained and food consumed was comparable across all groups throughout the study, with no statistically significant differences between IgG binding protein LT groups and controls in mean body weight gain or food consumption when compared over the dosing period (Dosing Days 1–91/92) and recovery period (Recovery Days 1–28).
3.4.3 Clinical Pathology
Clinical pathology parameters were unaffected by the administration of IgG binding protein LT. The only statistically significant differences in mean hematology and coagulation values for IgG binding protein LT groups compared with controls at the end of the dosing period were reticulocyte concentrations for females in all IgG binding protein LT groups (Table 5); however, these differences were considered to be unrelated IgG binding protein LT, as there was no clear dose–response relationship and all individual values were within historical control range (individual and historical control data not shown). Similarly for clinical chemistry (Table 6), there were only statistically significant differences between mean values for the IgG binding protein LT group versus controls in three of the analyzed parameters (creatine kinase was lower for females given 50- or 450-mg/kg body weight/day; globulin concentration was lower for females given 450-mg/kg body weight/day; and albumin:globulin ratio was higher for males given 50-mg/kg body weight/day) at the end of the dosing period; because none of the differences correlated with increasing dose, and all individual values were within historical control ranges, these differences were considered to be unrelated to IgG binding protein LT. At the end of the dosing period, urine volume and specific gravity were comparable across all groups, for both sexes (Table 7), and there were no effects of IgG binding protein LT on urinary pH, or on the presence of protein, glucose, ketones, bilirubin, blood, or sediment in urine (quantal data not shown).


3.4.4 Sperm Analysis and Estrous Cycles
There were no effects of IgG binding protein LT on the number of regular/irregular estrous cycles and mean cycle length nor were there any effects on any parameters related to the normal progression the spermatogenic cycle (including sperm count, motility, or morphology) at the end of the dosing period (data not shown).
3.4.5 Organ Weights and Macroscopic and Microscopic Examinations
Organ weights relative to body weight were unaffected by IgG binding protein LT (Table 8), with no statistically significant inter-group differences observed for any of the weighed organs. There were also no IgG binding protein LT–related macroscopic or histological abnormalities at necropsy; the only findings observed were considered incidental, as they were generally consistent with changes encountered in Wistar Han rats of this age kept under laboratory conditions (data not shown).


4 Discussion
IgG binding protein LT is likely to have an inherently low toxicity profile given its structural similarity with proteins that are naturally expressed in camel milk (which has a long history of consumption in East Africa and parts of Asia), its lack of systemic availability following oral consumption, and because it is produced using a generally recognized as safe organism (A. oryzae, which has been used for thousands of years in the production of fermented food products [e.g., saké, soy sauce, and miso] and has a long history of use in the production of food enzymes [Gomi 2014; Tu et al. 2025]); however, regulatory requirements dictate that the safety of novel ingredients must be comprehensively assessed before they may be marketed for use in foods and beverages. Therefore, in accordance with current regulatory guidelines, the assessment described in this research article included evaluations of the potential allergenicity, genotoxicity, and subchronic toxicity of IgG binding protein LT.



An internationally accepted approach to assessing the potential allergenicity of novel proteins is to predict cross-reactivity using a stepwise in silico approach, which considers that a protein has the potential for eliciting IgE cross-reactivity when a set of 80–amino acid length sequences (Segments 1–80, 2–81, 3–82, etc.) derived from the full-length amino acid sequence shares more than 35% identity with a known allergen, or if there is an exact match of six to eight contiguous amino acids (Codex Alimentarius 2003, 2009; FAO/WHO 2001). The in silico allergenicity searches described in this research article demonstrate that IgG binding protein LT does not share significant homology with known allergenic sequences, and the risk of it having the potential for cross-reactivity to known allergenic proteins is, therefore, very low. Predicting cross-reactivity using an exact match of six to eight contiguous amino acids and a default threshold of 35% identity over at least 80 amino acids is considered to be a highly conservative approach, as these searches have been reported to produce false positive results (EFSA GMO Panel 2022; Goodman et al. 2008; Hileman et al. 2002; Ladics 2019); therefore, these results provide a strong indication that IgG binding protein LT does not pose a significant allergenic risk to consumers. Approaches such as 3D protein structure similarity searches, and machine learning strategies based on IgE epitope matching, have been recently developed (EFSA GMO Panel 2022), and the use of these methods as additional complementary assessments to the traditional approach is a potential area for future research.
The potential genotoxicity of IgG binding protein LT was investigated using the bacterial reverse mutation assay and the in vitro mammalian cell micronucleus test, the standard Tier 1 genotoxicity test battery that covers the three major genetic endpoints (mutagenicity, clastogenicity, and aneugenicity) with the minimum number of tests (EFSA Scientific Committee 2011). The "treat and wash" procedure (commonly used when conducting this test with proteins, as the potential release of histidine and/or tryptophan into the bacterial cultures could lead to false positive results) was used for the bacterial reverse mutation assay (Thompson et al. 2005). The results of the in vitro genotoxicity tests conclusively demonstrate that IgG binding protein LT is nongenotoxic, which is to be expected, as there are no structural alerts for genotoxicity and the production process is not expected to introduce any genotoxic impurities.
A 90-day oral toxicity study (preceded by a 14-day dose range–finding study) was also conducted to assess the potential subchronic toxicity of IgG binding protein LT. In the 90-day study, there were no IgG binding protein LT–related deaths or clinical signs, no ocular changes, and no differences in motor activity or performance in the functional observation battery. Body weight, food consumption, clinical pathology parameters (hematology and coagulation, clinical chemistry, and thyroid hormones), sperm parameters, estrous cycles, and organ weights were also unaffected by IgG binding protein LT. Statistically significant differences between IgG binding protein LT groups and controls were only observed for a small minority of hematology and clinical chemistry parameters, but these differences were clearly unrelated to IgG binding protein LT administration, as they were generally not associated with a dose–response relationship and individual values were within historical control ranges, indicating that the values reflect normal biological variation. There were also no IgG binding protein LT–related macroscopic or histopathological findings. In absence of any IgG binding protein LT–related effects in this study, the highest dose tested (450-mg/kg body weight/day) was concluded to be the no-observed-adverse-effect level (NOAEL).

To determine an acceptable daily intake (ADI), a default margin of safety of 100-fold (10-fold for interspecies differences and 10-fold for intraspecies differences), which is widely accepted by several international scientific and regulatory agencies (COT 2007; FAO/WHO 2009b; US FDA 1993), is typically applied to a subchronic study's NOAEL, although the EFSA NDA Panel (2024) also applies an additional factor of 2 to extrapolate from subchronic to chronic exposure. Using the NOAEL from the 90-day study (450-mg/kg body weight/day) as the reference point, the ADI for IgG binding protein LT is as high as 4.5-mg/kg body weight/day for the general population (315 mg/day for a 70-kg adult) when using the default 100-fold margin of safety and is 2.25-mg/kg body weight/day (157.5 mg/day for a 70-kg adult) when applying the additional factor of 2 recommended by the EFSA NDA Panel (2024). Estimated high-level daily intakes of IgG binding protein LT from its proposed uses in food and beverage products (results of internal exposure assessments conducted using the National Health and Nutrition Examination Survey [2017–2018] for the US and EFSA's Dietary Exposure tool for the EU, data not shown) are well below these respective ADIs, demonstrating that IgG binding protein LT is safe for its proposed uses.
5 Conclusion
In silico allergenicity assessment results demonstrate that IgG binding protein LT is highly unlikely to pose a risk of allergenic cross-reactivity, as there were no matches with putative allergens in that assessment. This, in addition to the absence of any genotoxic potential of IgG binding protein LT in vitro and the lack of any IgG binding protein LT–related effects in the 90-day oral gavage toxicity study in rats, demonstrates the safety of IgG binding protein LT for its intended uses in foods and beverages for the general population.
Author Contributions
Kirt R. Phipps: writing – original draft, writing – review and editing, visualization. Sachin Patel: writing – original draft. Kevin Scaife: investigation, writing – original draft. Toby Holmes: methodology, investigation. Alica Šoltésová: methodology, investigation. Christel Jørgensen: writing – original draft, writing – review and editing, funding acquisition. Sandra Wingaard Thrane: writing – review and editing. Louise Kristine Vigsnæs: writing – review and editing. Nigel Baldwin: conceptualization, writing – review and editing, project administration, supervision.
Conflicts of Interest
Kirt R. Phipps, Kevin Scaife, and Sachin Patel are employees of Intertek Health Sciences Inc. Nigel Baldwin is an employee of Baldwin Advisory Services Ltd. Christel Jørgensen, Sandra Wingaard Thrane, and Louise Kristine Vigsnæs are employees of Bactolife A/S, which sponsored the studies reported in this research article. Intertek Health Sciences Inc. and Baldwin Advisory Services Ltd. have provided consultancy services to Bactolife A/S. Toby Holmes and Alica Šoltésová are employees of Labcorp Early Development Laboratories Ltd., where the in vitro and in vivo toxicological studies were conducted.
References
- Aalberse, R. C. 2000. "Structural Biology of Allergens." Journal of Allergy and Clinical Immunology 106, no. 2: 228–238. https://doi.org/10.1067/mai.2000.108434.
- Aardema, M. J., S. Albertini, P. Arni, et al. 1998. "Aneuploidy: A Report of an ECETOC Task Force." Mutation Research 410, no. 1: 3–79. https://doi.org/10.1016/s1383-5742(97)00029-x.
- Abdelmoteleb, M., C. Zhang, B. Furey, et al. 2021. "Evaluating Potential Risks of Food Allergy of Novel Food Sources Based on Comparison of Proteins Predicted From Genomes and Compared to www.AllergenOnline.org." Food and Chemical Toxicology 147: 111888. https://doi.org/10.1016/j.fct.2020.111888.
- Ait El Alla, O., Y. Zine-Eddine, S. Chaji, S. Boukrouh, K. Boutoial, and B. Faye. 2025. "Global Camel Milk Industry: A Comprehensive Overview of Production, Consumption Trends, Market Evolution, and Value Chain Efficiency." Small Ruminant Research 243: 107441. https://doi.org/10.1016/j.smallrumres.2025.107441.
- Arbabi-Ghahroudi, M. 2017. "Camelid Single-Domain Antibodies: Historical Perspective and Future Outlook." Frontiers in Immunology 8: 1589. https://doi.org/10.3389/fimmu.2017.01589.
- Breulmann, M., and B. Böer. 2010. "Camel Farms: A new Idea to Help Desert Ecosystems Recover." Rural21 44, no. 2: 39–40. https://www.rural21.com/fileadmin/_migrated/content_uploads/R21_Camel_farms_0210_01.pdf.
- Breulmann, M., B. Böer, U. Wernery, et al. 2007. The Camel From Tradition to Modern Times. A Proposal Towards Combating Desertification via the Establishment of Camel Farms Based on Fodder Production From Indigenous Plants and Halophytes. UNESCO Doha Office. https://unesdoc.unesco.org/ark:/48223/pf0000224033.
- Brown, A. P., N. Dinger, and B. S. Levine. 2000. "Stress Produced by Gavage Administration in the Rat." Contemporary Topics in Laboratory Animal Science 39, no. 1: 17–21.
- Bych, K., M. H. Mikš, T. Johanson, M. J. Hederos, L. K. Vigsnæs, and P. Becker. 2019. "Production of HMOs Using Microbial Hosts—From Cell Engineering to Large Scale Production." Current Opinion in Biotechnology 56: 130–137. https://doi.org/10.1016/j.copbio.2018.11.003.
- Cardoso, R. R., R. M. Santos, C. R. Cardoso, and M. O. Carvalho. 2010. "Consumption of Camel's Milk by Patients Intolerant to Lactose. A Preliminary Study." Revista Alergia México 57, no. 1: 26–32. https://camel-idee.com/wp-content/uploads/2020/04/Consumption-of-camel%E2%80%99s-milk-by-patients-intolerant-to-lactose-1.pdf.
- Codex Alimentarius. 2003. Guideline for the Conduct of Food Safety Assessment of Foods Produced Using Recombinant-DNA Microorganisms (CAC/GL 46-2003; Adopted in 2003, Annexes II and III Adopted in 2008). Codex Alimentarius. https://www.fao.org/fileadmin/user_upload/gmfp/resources/CXG_046e.pdf.
- Codex Alimentarius. 2009. Foods Derived From Modern Biotechnology. 2nd ed. Codex Alimentarius. https://www.fao.org/3/a1554e/a1554e00.htm.
- COT. 2007. Variability and Uncertainty in Toxicology of Chemicals in Food, Consumer Products and the Environment. Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT). https://cot.food.gov.uk/sites/default/files/cot/vutreportmarch2007.pdf.
- Damián, M. R., N. G. Cortes-Perez, E. T. Quintana, et al. 2022. "Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health." Microorganisms 10, no. 5: 1065. https://doi.org/10.3390/microorganisms10051065.
- Davani-Davari, D., M. Negahdaripour, I. Karimzadeh, et al. 2019. "Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications." Foods (Basel) 8, no. 3: 92. https://doi.org/10.3390/foods8030092.
- Dronkers, T. M. G., A. C. Ouwehand, and G. T. Rijkers. 2020. "Global Analysis of Clinical Trials with Probiotics." Heliyon 6, no. 7: e04467. https://doi.org/10.1016/j.heliyon.2020.e04467.
- EFSA ANS Panel. 2021. "Guidance for Submission for Food Additive Evaluations (EFSA Panel on Food Additives and Nutrient Sources Added to Food/ANS) (Question No EFSA-Q-2010-00675, Originally Adopted 7 June 2012 by European Food Safety Authority, Revision Endorsed 2 July 2020 by EFSA Food Additives and Flavourings Panel/ FAF for Implementation 27 March 2021)." EFSA Journal 10, no. 7: 2760. https://doi.org/10.2903/j.efsa.2012.2760.
- EFSA CEP Panel. 2021. "Scientific Guidance for the Submission of Dossiers on Food Enzymes (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids/CEP)(Question No: EFSA-Q-2020-00477, Adopted: 15 September 2021)." EFSA Journal 19: 6851. https://doi.org/10.2903/j.efsa.2021.6851.
- EFSA GMO Panel. 2017. "Guidance on Allergenicity Assessment of Genetically Modified Plants (EFSA Panel on Genetically Modified Organisms/GMO) (Question No: EFSA-Q-2014-00547, Adopted: 18 may 2017 by European Food Safety Authority)." EFSA Journal 15, no. 6: 4862. https://doi.org/10.2903/j.efsa.2017.4862.
- EFSA GMO Panel. 2022. "Scientific Opinion on Development Needs for the Allergenicity and Protein Safety Assessment of Food and Feed Products Derived From Biotechnology (EFSA Panel on Genetically Modified Organisms/GMO) (Question No: EFSA-Q-2020-00316, Adopted 2 December 2021)." EFSA Journal 20: 7044. https://doi.org/10.2903/j.efsa.2022.7044.
- EFSA NDA Panel. 2024. "Guidance on the Scientific Requirements for an Application for Authorisation of a Novel Food in the Context of Regulation (EU) 2015/2283 (EFSA Panel on Nutrition, Novel Foods and Food Allergens/NDA) (Question No: EFSA-Q-2023-00442, Adopted: 27 June 2024 by European Food Safety Authority)." EFSA Journal 22, no. 9: 8961. https://doi.org/10.2903/j.efsa.2024.8961.
- EFSA Scientific Committee. 2011. "Scientific Opinion on Genotoxicity Testing Strategies Applicable to Food and Feed Safety Assessment. (Question No EFSA-Q-2009-00782, Adopted: 13 September 2011, Last Updated: 3 October 2012 by European Food Safety Authority)." EFSA Journal 9, no. 9: 2379. https://doi.org/10.2903/j.efsa.2011.2379.
- Elhajoui, A., M. Cunha, and M. Kirsch-Volders. 1998. "Spindle Poisons Can Induce Polyploidy by Mitotic Slippage and Micronucleate Mononucleate in the Cytokinesis-Block Assay." Mutagenesis 13, no. 2: 193–198. https://doi.org/10.1093/mutage/13.2.193.
- Elison, E., L. K. Vigsnæs, L. Rindom Krogsgaard, et al. 2016. "Oral Supplementation of Healthy Adults With 2′-O-Fucosyllactose and Lacto-N-neotetraose is Well Tolerated and Shifts the Intestinal Microbiota." British Journal of Nutrition 116, no. 8: 1356–1368. https://doi.org/10.1017/S0007114516003354.
- FAO/WHO. 2001. Evaluation of Allergenicity of Genetically Modified Foods. Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO). https://www.fao.org/fileadmin/templates/agns/pdf/topics/ec_jan2001.pdf.
- FAO/WHO. 2009a. "4. Hazard Identification and Characterization: Toxicological and Human Studies. 4.10. Food Allergy and Other Food Hypersensitivities." In Principles and Methods for the Risk Assessment of Chemicals in Food (Environmental Health Criteria 240), 4.117-4.135. Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO). https://iris.who.int/bitstream/handle/10665/44065/WHO_EHC_240_7_eng_Chapter4.pdf?sequence=7.
- FAO/WHO. 2009b. "7. Risk Characterization." In Principles and Methods for the Risk Assessment of Chemicals in Food (Environmental Health Criteria 240), 7.1-7.21. Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO). https://iris.who.int/bitstream/handle/10665/44065/WHO_EHC_240_10_eng_Chapter7.pdf?sequence=10.
- Farah, Z. 2011. "Camel Milk." In Encyclopedia of Dairy Sciences, edited by J. W. Fuquay, P. F. Fox, and P. L. H. McSweeney, 2nd ed., 512–517. Elsevier.
- Farooq, U., S. Ahmed, G. Liu, et al. 2024. "Biochemical Properties of Sheep Colostrum and Its Potential Benefits for Human Health: A Review." Animal Biotechnology 35, no. 1: 2320726. https://doi.org/10.1080/10495398.2024.2320726.
- Fenech, M. 1998. "Important Variables That Influence Base-Line Micronucleus Frequency in Cytokinesis-Blocked Lymphocytes—A Biomarker for DNA Damage in Human Populations." Mutation Research 404, no. 1–2: 155–165. https://doi.org/10.1016/s0027-5107(98)00109-2.
- Fenech, M., S. Bonassi, J. Turner, et al. 2003. "Intra- and Inter-Laboratory Variation in the Scoring of Micronuclei and Nucleoplasmic Bridges in Binucleated Human Lymphocytes: Results of an International Slide-Scoring Exercise by the HUMN Project." Mutation Research 534, no. 1/2: 45–64. https://doi.org/10.1016/s1383-5718(02)00248-6.
- Fenech, M., N. Holland, W. P. Chang, E. Zeiger, and S. Bonassi. 1999. "The HUman MicroNucleus Project—An International Collaborative Study on the Use of the Micronucleus Technique for Measuring DNA Damage in Humans." Mutation Research 428, no. 1/2: 271–283. https://doi.org/10.1016/s1383-5742(99)00053-8.
- Fenster, K., B. Freeburg, C. Hollard, C. Wong, R. Rønhave Laursen, and A. C. Ouwehand. 2019. "The Production and Delivery of Probiotics: A Review of a Practical Approach." Microorganisms 7, no. 3: 83. https://doi.org/10.3390/microorganisms7030083.
- Ferreira, V. C., T. L. C. T. Barroso, L. E. N. Castro, R. G. da Rosa, and L. de Siqueira Oliveira. 2023. "An Overview of Prebiotics and Their Applications in Food Industry." European Food Research and Technology 249, no. 11: 2957–2976. https://doi.org/10.1007/s00217-023-04341-7.
- Fill, B. K., S. W. Thrane, M. Pichler, et al. 2022. "Orally Active Bivalent VL Construct Prevents Proliferation of Enterohemorrhagic Escherichia coli in Weaned Piglets." iScience 25, no. 4: 104003. https://doi.org/10.1016/j.isci.2022.104003.
- Gapper, L. W., D. E. Copestake, D. E. Otter, and H. E. Indyk. 2007. "Analysis of Bovine Immunoglobulin G in Milk, Colostrum and Dietary Supplements: A Review." Analytical and Bioanalytical Chemistry 389, no. 1: 93–109. https://doi.org/10.1007/s00216-007-1391-z.
- Gibson, G. R., R. Hutkins, M. E. Sanders, et al. 2017. "Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics." Nature Reviews Gastroenterology & Hepatology 14, no. 8: 491–502. https://doi.org/10.1038/nrgastro.2017.75.
- Gomi, K. 2014. "Aspergillus/Aspergillus oryzae." In Encyclopedia of Food Microbiology, edited by C. A. Batt and M. L. Tortorello, 2nd ed., 92–96. Academic Press.
- Goodman, R. E., S. Vieths, H. A. Sampson, et al. 2008. "Allergenicity Assessment of Genetically Modified Crops—What Makes Sense? (Erratum published in Nature Biotechnology 26, no. 1: 73–81. https://doi.org/10.1038/nbt1343.
- Grand View Research. 2022. *Functional Foods Market Size, Share & Trends Analysis Report by Ingredient (Carotenoids, Prebiotics & Probiotics, Fatty Acids, Dietary Fibers), by Product, by Application, by Region, and Segment Forecasts, 2022–2030 (Report ID: GVR-1-68038-195-5). Grand View Research. https://www.grandviewresearch.com/industry-analysis/functional-food-market.
- Hamers-Casterman, C., T. Atarhouch, S. Muyldermans, et al. 1993. "Naturally Occurring Antibodies Devoid of Light Chains." Nature 363, no. 6428: 446–448. https://doi.org/10.1038/363446a0.
- Harmsen, M. M., C. B. van Solt, and H. P. Fijten. 2009. "Enhancement of Toxin- and Virus-Neutralizing Capacity of Single-Domain Antibody Fragments by N-Glycosylation." Applied Microbiology and Biotechnology 84, no. 6: 1087–1094. https://doi.org/10.1007/s00253-009-2091-1.
- Hileman, R. E., A. Silvanovich, R. E. Goodman, et al. 2002. "Bioinformatic Methods for Allergenicity Assessment Using a Comprehensive Allergen Database." International Archives of Allergy and Immunology 128, no. 4: 280–291. https://doi.org/10.1159/000063861.
- Hurley, W. L., and P. K. Theil. 2011. "Perspectives on Immunoglobulins in Colostrum and Milk." Nutrients 3, no. 4: 442–474. https://doi.org/10.3390/nu3040442.
- Jenkins, T. P., N. Ács, E. W. Arendrup, et al. 2024. "Protecting the Piglet Gut Microbiota Against ETEC-Mediated Post-Weaning Diarrhoea Using Specific Binding Proteins." npj Biofilms and Microbiomes 10, no. 1: 42. https://doi.org/10.1038/s41522-024-00512-7.
- Kessler, E. C., R. M. Bruckmaier, and J. J. Gross. 2019. "Immunoglobulin G Content and Colostrum Composition of Different Goat and Sheep Breeds in Switzerland and Germany." Journal of Dairy Science 102, no. 6: 5542–5549. https://doi.org/10.3168/jds.2018-16231.
- Konuspayeva, G., and B. Faye. 2021. "Recent Advances in Camel Milk Processing." Animals 11, no. 4: 1045. https://doi.org/10.3390/ani11041045.
- Ladics, G. S. 2019. "Assessment of the Potential Allergenicity of Genetically-Engineered Food Crops." Journal of Immunotoxicology 16, no. 1: 1–3. https://doi.org/10.1080/1547691X.2018.1547559.
- Mbogo, E. N., C. R. Field, K. J. Ngeitywa, and K. A. Abey. 2012. "Introduction [and] Chapter 1: Origin and Uses of Camels." In Camel Manual for Kenya, edited by N. Younan, M. Zaidi, and D. Sikuku, 1–10. Kenya Camel Association and Kenya Agricultural Research Institute. https://infonet-biovision.org/sites/default/files/pdf/2012june_camel_manual.pdf.
- Mehra, R., and P. Kelly. 2006. "Milk Oligosaccharides: Structural and Technological Aspects." International Dairy Journal 16, no. 11: 1334–1340. https://doi.org/10.1016/j.idairyj.2006.06.008.
- Migliore, L., and M. Nieri. 1991. "Evaluation of Twelve Potential Aneuploidogenic Chemicals by the In Vitro Human Lymphocyte Micronucleus Assay." Toxicology In Vitro 5, no. 4: 325–336. https://doi.org/10.1016/0887-2333(91)90006-3.
- Miller, B., F. Pötter-Locher, A. Seelbach, H. Stopper, D. Utesch, and S. Madle. 1998. "Evaluation of the In Vitro Micronucleus Test as an Alternative to the In Vitro Chromosomal Aberration Assay: Position of the GUM Working Group on the In Vitro Micronucleus Test. Gesellschaft für Umwelt-Mutations-Forschung." Mutation Research 410, no. 1: 81–116. https://doi.org/10.1016/s1383-5742(97)00030-6.
- Morrin, S. T., R. H. Buck, M. Farrow, and R. M. Hickey. 2021. "Milk-Derived Anti-Infectives and Their Potential to Combat Bacterial and Viral Infection." Journal of Functional Foods 81: 104442. https://doi.org/10.1016/j.jff.2021.104442.
- Muyldermans, S. 2013. "Nanobodies: Natural Single-Domain Antibodies." Annual Review of Biochemistry 82: 775–797. https://doi.org/10.1146/annurev-biochem-063011-092449.
- Ngeitywa, K. J., and J. C. Njanja. 2013. "Advocacy for Camel Research and Development in Kenya." Ethiopian Journal of Agricultural Sciences 7, no. 5: 539–546. https://doi.org/10.17265/1934-7391/2013.05.015.
- Nguyen, M. N., N. L. Krutz, V. Limviphuvadh, A. L. Lopata, G. F. Gerberick, and S. Maurer-Stroh. 2022. "AllerCatPro 2.0: A Web Server for Predicting Protein Allergenicity Potential." Nucleic Acids Research 50, no. W1: W36–W43. https://doi.org/10.1093/nar/gkac446.
- OECD. 1997. "Bacterial Reverse Mutation Test." In OECD Guidelines for the Testing of Chemicals (OECD Guideline, No. 471). Organisation for Economic Co-operation and Development (OECD). https://doi.org/10.1787/9789264071247-en.
- OECD. 1998. OECD Principles of Good Laboratory Practice (Series on Principles of Good Laboratory Practice and Compliance Monitoring, No. 1). Organisation for Economic Co-operation and Development (OECD). https://doi.org/10.1787/9789264078536-en.
- OECD. 2016. "In Vitro Mammalian Cell Micronucleus Test." In OECD Guidelines for the Testing of Chemicals (OECD Guideline, No. 487). Organisation for Economic Co-operation and Development (OECD). https://doi.org/10.1787/9789264264861-en.
- OECD. 2018. "Repeated Dose 90-Day Oral Toxicity Study in Rodents." In OECD Guidelines for the Testing of Chemicals (OECD Guideline, No. 408). Organisation for Economic Co-operation and Development (OECD). https://doi.org/10.1787/9789264070707-en.
- Palsson, O. S., A. Peery, D. Seitzberg, I. D. Amundsen, B. McConnell, and M. Simren. 2020. "Human Milk Oligosaccharides Support Normal Bowel Function and Improve Symptoms of Irritable Bowel Syndrome: A Multicenter, Open-Label Trial." Clinical and Translational Gastroenterology 11, no. 12: e00276. https://doi.org/10.14309/ctg.0000000000000276.
- Percie du Sert, N., V. Hurst, A. Ahluwalia, et al. 2020. "The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research." PLoS Biology 18, no. 7: e3000410. https://doi.org/10.1371/journal.pbio.3000410.
- Petersson, M., S. W. Thrane, L. Gram, S. Muyldermans, and A. H. Laustsen. 2023. "Orally Delivered Single-Domain Antibodies against Gastrointestinal Pathogens." Trends in Biotechnology 41, no. 7: 875–886. https://doi.org/10.1016/j.tibtech.2023.01.015.
- Petschow, B., J. Burnett, A. L. Shaw, F. M. Weaver, and G. J. Klein. 2014. "Serum-Derived Bovine Immunoglobulin/Protein Isolate: Postulated Mechanism of Action for Management of Enteropathy." Clinical and Experimental Gastroenterology 7: 181–190. https://doi.org/10.2147/ceg.S62823.
- Proliant Health Ingredients Inc. 2008. GRAS Notification for ImmunoLin™ Brand Spray-Dried Bovine Globulin Concentrate Intended for Use as an Ingredient in Foods (GRAS Notice GRN No. 255). Proliant Health Ingredients Inc. Notified US Food and Drug Administration (US FDA). https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=255.
- Ramani, A., A. Taherabbas, and S. Manik. 2024. "Bovine Colostrum as a Promising Nutraceutical: A Systematic Review." Sustainable Food Technology 2, no. 3: 531–547. https://doi.org/10.1039/D3FB00080J.
- Richardson, C., D. A. Williams, J. A. Allen, G. Amphlett, D. O. Chanter, and B. Phillips. 1989. "Analysis of Data From In Vitro Cytogenetic Assays." In Statistical Evaluation of Mutagenicity Test Data, UKEMS Guidelines (Subcommittee Report, Part III) edited by D. J. Kirkland and G. A. T. Mahon, 141–154. Cambridge University Press.
- Rosefort, C., E. Fauth, and H. Zankl. 2004. "Micronuclei Induced by Aneugens and Clastogens in Mononucleate and Binucleate Cells Using Cytokinesis Block Assay." Mutagenesis 19, no. 4: 277–284. https://doi.org/10.1093/mutage/geh028.
- Ryan, J. J., A. Monteagudo-Mera, N. Contractor, and G. R. Gibson. 2021. "Impact of 2′-Fucosyllactose on Gut Microbiota Composition in Adults With Chronic Gastrointestinal Conditions: Batch Culture Fermentation Model and Pilot Clinical Trial Findings." Nutrients 13, no. 3: 938. https://doi.org/10.3390/nu13030938.
- Sanders, M. E., D. J. Merenstein, G. Reid, G. R. Gibson, and R. A. Rastall. 2019. "Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic (Correction, Nature Reviews. Gastroenterology & Hepatology 16(10), 642)." Nature Reviews. Gastroenterology & Hepatology 16, no. 10: 605–616. https://doi.org/10.1038/s41575-019-0173-3.
- Scaife, K., S. L. Taylor, L. Pařenicová, et al. 2024. "In Silico Evaluation of the Potential Allergenicity of a Fungal Biomass From Rhizomucor pusillus for Use as Novel Food Ingredient." Regulatory Toxicology and Pharmacology 150: 105629. https://doi.org/10.1016/j.yrtph.2024.105629.
- Schönknecht, Y. B., M. V. Moreno Tovar, S. R. Jensen, and K. Parschat. 2023. "Clinical Studies on the Supplementation of Manufactured Human Milk Oligosaccharides: A Scoping Review." Nutrients 15, no. 16: 3622. https://doi.org/10.3390/nu15163622.
- Schroeder, H. W., Jr., and L. Cavacini. 2010. "Structure and Function of Immunoglobulins." Journal of Allergy and Clinical Immunology 125, no. 2 Suppl 2: S41–S52. https://doi.org/10.1016/j.jaci.2009.09.046.
- Seifu, E. 2009. "Analysis of the Contributions of and Constraints to Camel Production in Shinile and Jijiga Zones, Eastern Ethiopia." Journal of Agriculture and Rural Development in the Tropics and Subtropics (JÄEID) 103, no. 3: 213–224. https://doi.org/10.12895/jaeid.20093.33.
- Smits, M. G., T. Huppertz, A. C. Alting, and J. Kiers. 2011. "Composition, Constituents and Properties of Dutch Camel Milk." Journal of Camel Practice and Research 18, no. 1: 1-7. Available at: https://www.indianjournals.com/ijor.aspx?target=ijor:jcpr&volume=18&issue=1&article=001.
- Swelum, A. A., M. T. El-Saadony, M. Abdo, et al. 2021. "Nutritional, Antimicrobial and Medicinal Properties of Camel's Milk: A Review." Saudi Journal of Biological Sciences 28, no. 5: 3126–3136. https://doi.org/10.1016/j.sjbs.2021.02.057.
- Thompson, C., P. Morley, D. Kirkland, and R. Proudlock. 2005. "Modified Bacterial Mutation Test Procedures for Evaluation of Peptides and Amino Acid-Containing Material." Mutagenesis 20, no. 5: 345–350. https://doi.org/10.1093/mutage/gei045.
- Thybaud, V., M. Aardema, J. Clements, et al. 2007. "Strategy for Genotoxicity Testing: Hazard Identification and Risk Assessment in Relation to In Vitro Testing." Mutation Research 627, no. 1: 41–58. https://doi.org/10.1016/j.mrgentox.2006.10.003.
- Tripathi, N. K., and A. Shrivastava. 2019. "Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development." Frontiers in Bioengineering and Biotechnology 7: 420. https://doi.org/10.3389/fbioe.2019.00420.
- Tu, Z., D. Choi, Y. Chen, J. H. Yu, and T. N. Huynh. 2025. "The Food Fermentation Fungus Aspergillus oryzae Is a Source of Natural Antimicrobials Against Listeria monocytogenes." Journal of Dairy Science 108, no. 4: 3444–3454. https://doi.org/10.3168/jds.2024-25719.
- Turner, P. V., T. Brabb, C. Pekow, and M. A. Vasbinder. 2011. "Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider." Journal of the American Association for Laboratory Animal Science: JAALAS 50, no. 5: 600–613.
- UK Government. 1999. The Good Laboratory Practice Regulations 1999 (UK Statutory Instruments, 1999 No. 3106). His Majesty's Stationary Office (HMSO). https://www.legislation.gov.uk/uksi/1999/3106/contents/made.
- UK Government. 2012. The Animals (Scientific Procedures) Act 1986. Amendment Regulations 2012. His Majesty's Stationary Office (HMSO). https://www.legislation.gov.uk/ukdsi/2012/9780111530313.
- UK Government. 2014. Code of Practice for the Housing and Care of Animals Bred, Supplied or Used for Scientific Purposes. His Majesty's Stationary Office (HMSO). https://assets.publishing.service.gov.uk/media/5a7ee67ce5274a2e8ab48e98/CoOAnimalsWeb.pdf.
- Ulfman, L. H., J. H. W. Leusen, H. F. J. Savelkoul, J. O. Warner, and R. J. J. van Neerven. 2018. "Effects of Bovine Immunoglobulins on Immune Function, Allergy, and Infection." Frontiers in Nutrition 5: 52. https://doi.org/10.3389/fnut.2018.00052.
- US FDA. 1993. "Chapter II. Agency Review of Toxicology Information in Petitions for Direct Food Additives and Color Additives Used in Food." In Toxicological Principles for the Safety Assessment of Direct Food Additives and Color Additives Used in Food. Redbook II (Draft Guidance). US Food and Drug Administration (US FDA). https://www.fda.gov/media/71274/download#attachment.
- US FDA. 2007. "Toxicological Principles for the Safety Assessment of Food Ingredients." In Redbook 2000 (Guidance for Industry and Other Stakeholders). US Food and Drug Administration (US FDA). https://www.fda.gov/food/guidance-regulation-food-and-dietary-supplements/guidance-industry-and-other-stakeholders-redbook-2000.
- US FDA. 2024. "Part 1240—Control of Communicable Diseases. §1240.61—Mandatory Pasteurization for All Milk and Milk Products in Final Package Form Intended for Direct Human Consumption." In 21 Code of Federal Regulations (CFR). Title 21—Food and Drugs (Food and Drug Administration), US Government Publishing Office (GPO). https://www.govinfo.gov/app/details/CFR-2024-title21-vol8/CFR-2024-title21-vol8-sec1240-61.
- Wilson, R. T. 1998. Camels: The Tropical Agriculturalist. Macmillan Education Ltd.
- Winer, B. J. 1971. Statistical Principles in Experimental Design. 2nd ed. McGraw-Hill.
- Yakhkeshi, S., R. Wu, B. Chelliappan, and X. Zhang. 2022. "Trends in Industrialization and Commercialization of IgY Technology." Frontiers in Immunology 13: 991931. https://doi.org/10.3389/fimmu.2022.991931.
Data Availability Statement
Research data are not shared.